Abstract
Neurodegenerative diseases (NDDs) are complex disorders that degenerates central nervous system. To this end, we have achieved only palliative treatments and their success is limited. Emerging studies suggest stem cells could be an alternative to recover lost neural network. Transplanting stem cells for replacing damaged neurons is a pivotal step in cell replacement therapies. In this article, NDDs and their pathology, current methods of combating NDDs and potentiality of stem cells in treating NDDs have been reviewed briefly. In addition to this , technical issues that hamper clinical applications of stem cells in creating cellular models and grafted cells for neuron resurrection have been discussed.
Introduction
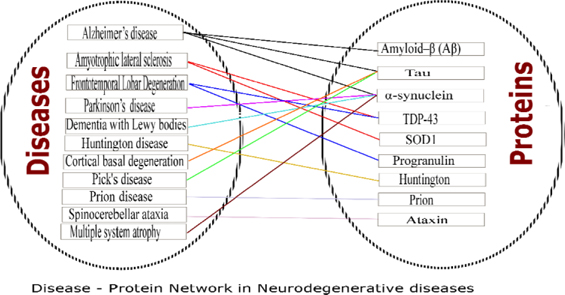
Neurodegenerative diseases (NDDs) are devastating disorders with complex etiology. Loss of neurons in the central nervous system, which leads to dementia/ataxia or both is the characteristic feature of NDDsSanchez-Mut et al., 2016Uttara et al., 2009. The pattern in which they occur slightly vary from disease to disease, yet all ultimately culminate to the progressive degeneration of neurons. However, the mechanism which commences this degradation is not clear. Many neurodegenerative diseases are characterized by the aggregation of misfolded/ abnormal proteins, along with fibril formation and depositions. The Figure 1 shows the diseases in NDDs and observed pathological proteins.
These diseases are inexorable and there is a need for exigent solution for this crisis. Inefficacy of present treatments which are solely symptomatic, necessitate alternative approaches to combat with higher efficiency and to provide long term solution.
Phenomenal properties of stem cells and possibility of applications in regenerative medicine, encourages patient-oriented studies in neurodegenerative diseases. Stem cells are considered as cellular models to investigate disease pathology as well as transplantable grafts to recover, to ameliorate and to protect nervous system becomes intriguing research Abud and Blurton-Jones, 2016Haston and Finkbeiner, 2016Wojda and Kuznicki, 2013.
This article aims to address recent advances of stem cells in NDDs for developing disease models and repairing neuron loss. This article will serve as primer to understand potential of stem cells in neurodegenerative resurrection. However, for deep insights on pathology and treatment methodologies, further reading on cited articles is inevitable. Among various neurodegenerative diseases, this article will focus on Alzheimer’s disease, Parkinson’s disease, Amyotrophic lateral sclerosis and Frontotemporal lobar degeneration.
Neurodegenerative diseases (ndds)
Alzheimer’s disease (AD): It is the most common and prevalent NODs. In 2015, 46.8 million people reported to have dementia, out of which over 60 percent of dementia are due to AD Prince et al., 2016 5% of AD are Familial AD (FAD) and over 95 % of AD are Sporadic AD (SAD) Hunter et al., 2013. AD is characterized by the degeneration of neurons in basal forebrain, amygdala, hippocampus and cortical area, culminating in perceivable declination of memory and other cognitive functions such as thinking, understanding and attention Whitehouse et al., 1981. Formation of amyloid- β peptide (A β) plaques and neurofibrillary tangles (Waldau and Shetty, 2008) are ideal hallmarks of the disease. Drugs inhibiting formations A β plaques and neurofibrillary tangles are used in symptomatic treatment.
Parkinson’s disease (PD)
Parkinson’s disease is the second most common cause of progressive NODs. Worldwide, 10 million people are affected by PD (http://www.pdf.org/en/parkinson_statistics.2013). PD is characterized by the degeneration of nigrostriatal dopaminergic neurons Dirnberger and Jahanshahi, 2013. Extensive degeneration of dopaminergic neurons results in programmed cell death, viral infection and accumulation of environmental toxins Lang and Lozano, 1998. Hallmarks of pathogenesis are protein mis-folding and dysfunction of ubiquitin proteasome pathway. Lewy bodies formation occurs with α-synuclein and ubiquitin. Symptoms include bradykinesia, rigidity, resting tremor and postural instability. It is also associated with non-motor symptoms Dauer and Przedborski,2003.
Frontotemporal lobar degeneration (FTLD) and Amyotrophic lateral sclerosis (ALS)
Frontotemporal lobar degeneration (FTLD) and Amyotrophic lateral sclerosis (ALS) are most commonly overlapping neurodegenerative diseases. FTLD is a non-Alzheimer form of dementia characterized by either abnormal behavioral or aphasic patterns in people under the age of 65 years Neary et al., 1998. FTLD can be sporadic or familial. FTLD can occur independently or with combination of amyotrophic lateral sclerosis, Parkinson’s disease and with other neurodegenerative diseases Ratnavalli et al., 2002. Amyotrophic lateral sclerosis is a neurodegenerative motor neuron disease. It is also called as Lou Gehrig’s disease. It affects both upper and lower motor neurons. Upper motor neuron degeneration signs are hyperreflexia, extensor plantar response, increased muscle tone and weakness in topographic representation, while lower motor neuron degeneration signs comprise of weakness, muscle wasting, muscle cramps, fasciculation’s and hyporeflexia. Regardless of initial symptoms, it eventually results in muscle atrophy and weakness Hardiman et al., 2011. Death occurs within 3 to 5 years of diagnosis. Only 10% of ALS is familial, while 90% is sporadic. Genetic reasons for ALS still remain unclear AI-Chalabi et al., 2012.
FTLD and ALS are caused as a result of dysfunction of many proteins. Origin of these neuro-degenerative diseases are multifactorial, among the numerous proteins such as Tau, Tar DNA binding (TDP-43) protein and Fused in Sarcoma (FUS) protein dysfunctions are observed commonly in both FTLD and ALS. Biological properties of these proteins are different in normal and diseased conditions. For instance, in healthy people TDP-43 is localized in the nucleus and it plays a role in transcriptional regulation, pre-mRNA splicing, microRNA processing and mRNA transport (Buratti, 2(08). Contradictorily, in FTLD and ALS patients, TDP-43 proteins are present as aggregates in the cytoplasm.
Stem Cells in NDDs
Cells that possess the property of self-renewal and differentiation to multiple cell types are called stem cells. Stem cells are classified into the following types based on their ability to differentiate.
1) Totipotent stem cells, that differentiate to any type of cell (e.g. Embryonic four cell stage)
2) Pluripotent stem cells, that differentiate to many cell types (e.g. Embryonic stem cells)
3) Multipotent stem cells differentiation are limited to few cell types (e.g. Adult stem cells)
Induced Pluripotent cells (iPSCs). These cells are non pluripotent cells (somatic cells) which are induced to become pluripotent. This induction via regulating transcription factors such as Oct4, Sox2, Klf4 and c-Myc Eminli et al., 2008Okita et al., 2007Wernig et al., 2007
Embryonic stem cells (ESCs), Mesenchymal stem cells (MSCs) and Induced pluripotent stem cells (iPSCs) are transformed to neuronal progenitor cells (NPCs) or neuronal stem cells (NSCs) from which several neuronal lineages are obtained based on specific differentiation. Upshot is that, reprogramming the cell to various neuronal lineage to correct neurodegenerative diseases. Manipulating expression of the transcription factors will convert fibroblast cells to neuronal cells. Factors such as Asell, Brn2, and Myillin Pang et al.,2011 and Mashl, Ngn2, Nurrl and Ptx3 in Liu et al., 2012 reported to reprogram the cells to neuronal cells.
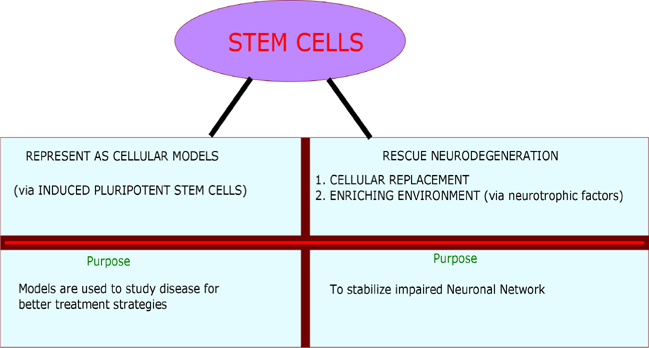
Induced pluripotent stem cells were used in developing cellular models. iPSCs were used to create models for AD Israel et al., 2012 PD (Woodard et al., 2014), ALS Egawa et al., 2012 and Huntington’s disease Juopperi et al., 2012. These models assist in understanding the pathological changes occurred during the disease progression. These changes could be of phenotypic or sub cellular observations Swinney and Anthony, 2011. Apart from developing cellular models, stem cells are used in two ways to treat neurodegenerative diseases. In first place, stem cells are "Necessary cell population" in transplanted affected region, where it is intended to replace the complete functionality of degenerated neurons. Secondly, stem cells are used as "Auxiliary cell population" to serve as a source to induce other cells via promoting the secretion of neuroprotective growth factors such as brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), vascular endothelial growth factor (VEGF) and insulin-like growth factor-1 (IGF-1) Medvedev et al., 2010Suzuki and Svendsen, 2008Xuan et al., 2008. Figure 2 shows the application of stem cells in NDDs.
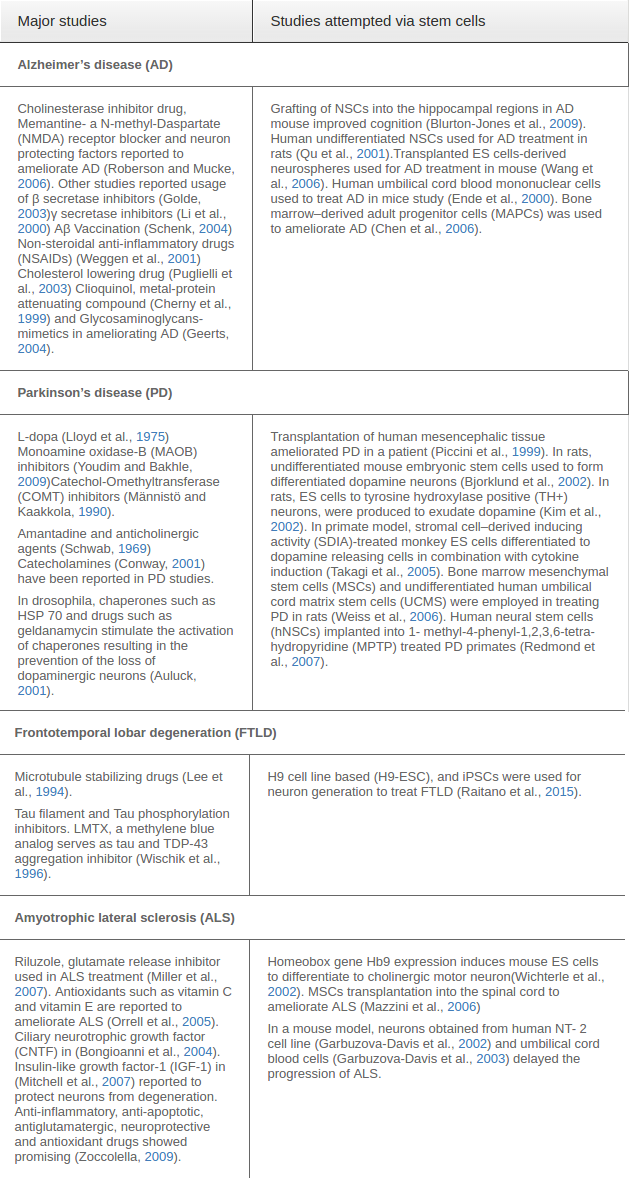
Representative studies of currently available studies in treating NDDs and potentiality of stem cell in treating NDDs have been tabulated in Table 1 .
Conclusions
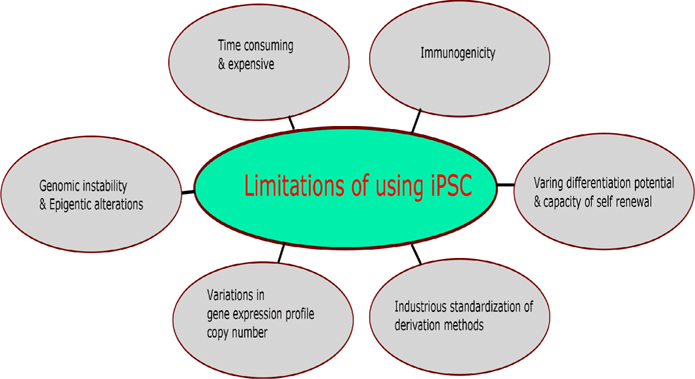
Review by Avior et al., 2016Yamanaka, 2012 discussed pros and cons of application of pluripotent stem eells in disease modeling and disease rescuing approaches. Figure 3 was drawn based on Avior et al., 2016Yamanaka, 2012 shows the limitations of pluripotent stem cells. Also, the application of stem cells in the resurrection of neurodegenerative diseases is hampered by limited understanding in
1) Quantitative determination of fate of transplanted neuronal cells in integration, synaptic connection and recapitulating in the neuronal network remains unanswered.
2) Docs introduced neurons into the brain cause tumorigenesis?
3) Does introduced neurons sluvive and reinnervate and what determines its efficacy?
4) To what extent axon has to grow in the affected regions and what regulates its migration are must to know information for effective stem cell based therapies.
To date, advancement in stem cell therapies in improving treabnents in neurodegcnerative diseases are promising. However, it is too early to vouch for stem cell based therapies in humans.
Abbreviations
AD: Alzheimer’s disease. ALS: Amyotrophic lateral sclerosis. BDNF: Brain- derived neurotrophic factor. CNTF: Ciliary neurotrophic growth factor. COMT: Catechol-O-methyltransferase. ESCs: EmbryoniC stem cells. FAD: Familial Alzheimer’s disease. FTLD: Frontotemporal lobar degeneration. FUS: Fused in Sarcoma. GDNF: Glial-derived neurotrophic factor. hNSCs: Human neural stem cells. HSP 70: Heat shock protein 70. IGF-I: Insulin-like growth factor-I. iPSCs: Induced Pluripotent cells. L-DOPA: L-3, 4dihydroxyphenylalanine. MAO-B: Monoamine oxidase-B. MAPCs: Marrow-derived adult progenitor cells. MPTP: I-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. MSCs: Mesenchymal stem cells, NDDs: Neurodegenerative diseases. NMDA: Nmethyl- D-aspartate. NSAIDs: Non-steroidal antiinflammatory drugs. NSCs: Neuronal stem cells. PD: Parkinson’s disease. SAD: Sporadic Alzheimer’s disease. SDIA: Stromal cell-derived inducing activity. TDP-43: Tar DNA binding protein- 43. UCMS: Umbilical cord matrix stem cells. VEGF: Vascular endothelial growth factor.
References
-
E.M.
Abud,
M.
Blurton-Jones.
Could Stem Cells Be Used to Treat or Model Alzheimer’s Disease?. In Translational Neuroscience (Springer Science + Business Media).
2016;
:
203-225
.
-
A.
Al-Chalabi,
A.
Jones,
C.
Troakes,
A.
King,
S.
Al-Sarraj,
L.H.
van den Berg.
The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathologica.
2012;
124
:
339-352
.
-
P.K.
Auluck.
Chaperone Suppression of alpha -Synuclein Toxicity in a Drosophila Model for Parkinson’s Disease. Science.
2001;
295
:
865-868
.
-
Y.
Avior,
I.
Sagi,
N.
Benvenisty.
Pluripotent stem cells in disease modelling and drug discovery. ature Reviews Molecular Cell Biology.
2016;
17
:
170-182
.
-
L.M.
Bjorklund,
R.
Sanchez-Pernaute,
S.
Chung,
T.
Andersson,
I.Y.C.
Chen,
K.S.P.
McNaught,
A.L.
Brownell,
B.G.
Jenkins,
C.
Wahlestedt,
K.S.
Kim.
Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proceedings of the National Academy of Sciences.
2002;
99
:
2344-2349
.
-
M.
Blurton-Jones,
M.
Kitazawa,
H.
Martinez-Coria,
N.A.
Castello,
F.J.
Muller,
J.F.
Loring,
T.R.
Yamasaki,
W.W.
Poon,
K.N.
Green,
F.M.
LaFerla.
Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proceedings of the National Academy of Sciences.
2009;
106
:
13594-13599
.
-
P.
Bongioanni,
C.
Reali,
V.
Sogos.
Ciliary neurotrophic factor (CNTF) for amyotrophic lateral sclerosis or motor neuron disease. In Cochrane Database of Systematic Reviews (Wiley-Blackwell).
2004
.
-
E.
Buratti.
Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Frontiers in Bioscience.
2008;
13
:
867
.
-
C.-W.
Chen,
R.
Boiteau,
W.-F.
Lai,
S.
Barger,
A.
Cataldo.
sAPP Enhances the Transdifferentiation of Adult Bone Marrow Progenitor Cells to Neuronal Phenotypes. CAR.
2006;
3
:
63-70
.
-
R.A.
Cherny,
J.T.
Legg,
C.A.
McLean,
D.P.
Fairlie,
X.
Huang,
C.S.
Atwood,
K.
Beyreuther,
R.E.
Tanzi,
C.L.
Masters,
A.I.
Bush.
Aqueous Dissolution of Alzheimer’s Disease A Amyloid Deposits by Biometal Depletion. Journal of Biological Chemistry.
1999;
274
:
23223-23228
.
-
K.A.
Conway.
Kinetic Stabilization of the alpha -Synuclein Protofibril by a Dopamine-alpha -Synuclein Adduct. Science.
2001;
294
:
1346-1349
.
-
W.
Dauer,
S.
Przedborski.
Parkinson’s Disease. Neuron.
2003;
39
:
889-909
.
-
G.
Dirnberger,
M.
Jahanshahi.
Executive dysfunction in Parkinson’s disease: A review. Journal of Neuropsychology.
2013;
7
:
193-224
.
-
N.
Egawa,
S.
Kitaoka,
K.
Tsukita,
M.
Naitoh,
K.
Takahashi,
T.
Yamamoto,
F.
Adachi,
T.
Kondo,
K.
Okita,
I.
Asaka.
Drug Screening for ALS Using Patient-Specific Induced Pluripotent Stem Cells. Science Translational Medicine.
2012;
4
:
145ra104-145ra104
.
-
S.
Eminli,
J.
Utikal,
K.
Arnold,
R.
Jaenisch,
K.
Hochedlinger.
Reprogramming of Neural Progenitor Cells into Induced Pluripotent Stem Cells in the Absence of Exogenous Sox2 Expression. STEM CELLS.
2008;
26
:
2467-2474
.
-
N.
Ende,
F.
Weinstein,
R.
Chen,
M.
Ende.
Human umbilical cord blood effect on sod mice (amyotrophic lateral sclerosis). Life Sciences.
2000;
67
:
53-59
.
-
S.
Garbuzova-Davis,
A.E.
Willing,
M.
Milliken,
S.
Saporta,
T.
Zigova,
D.W.
Cahill,
P.R.
Sanberg.
Positive Effect of Transplantation of hNT Neurons (NTera 2/D1 Cell-Line) in a Model of Familial Amyotrophic Lateral Sclerosis. Experimental Neurology.
2002;
174
:
169-180
.
-
S.
Garbuzova-Davis,
A.E.
Willing,
T.
Zigova,
S.
Saporta,
E.B.
Justen,
J.C.
Lane,
J.E.
Hudson,
N.
Chen,
C.D.
Davis,
P.R.
Sanberg.
Intravenous Administration of Human Umbilical Cord Blood Cells in a Mouse Model of Amyotrophic Lateral Sclerosis: Distribution, Migration, and Differentiation. Journal of Hematotherapy & Stem Cell Research.
2003;
12
:
255-270
.
-
H.
Geerts.
NC-531 (Neurochem). Current opinion in investigational drugs (London, England: 2000).
2004;
5
:
95
.
-
T.E.
Golde.
Alzheimer disease therapy: Can the amyloid cascade be halted?. Journal of Clinical Investigation.
2003;
111
:
11-18
.
-
O.
Hardiman,
L.H.
van den Berg,
M.C.
Kiernan.
Clinical diagnosis and management of amyotrophic lateral sclerosis. Nature Reviews Neurology.
2011;
7
:
639-649
.
-
K.M.
Haston,
S.
Finkbeiner.
Clinical Trials in a Dish: The Potential of Pluripotent Stem Cells to Develop Therapies for Neurodegenerative Diseases. Annu Rev Pharmacol Toxicol.
2016;
56
:
489-510
.
-
S.
Hunter,
T.
Arendt,
C.
Brayne.
The Senescence Hypothesis of Disease Progression in Alzheimer Disease: an Integrated Matrix of Disease Pathways for FAD and SAD. Molecular Neurobiology.
2013;
48
:
556-570
.
-
M.A.
Israel,
S.H.
Yuan,
C.
Bardy,
S.M.
Reyna,
Y.
Mu,
C.
Herrera,
M.P.
Hefferan,
S.
Van Gorp,
K.L.
Nazor,
F.S.
Boscolo.
Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature.
2012
.
-
T.A.
Juopperi,
W.
Kim,
C.-H.
Chiang,
H.
Yu,
R.L.
Margolis,
C.A.
Ross,
G.-l.
Ming,
H.
Song.
Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Molecular Brain.
2012;
5
:
17
.
-
J.-H.
Kim,
J.M.
Auerbach,
J.A.
Rodríguez-Gómez,
I.
Velasco,
D.
Gavin,
N.
Lumelsky,
S.-H.
Lee,
J.
Nguyen,
R.
Sánchez- Pernaute,
K.
Bankiewicz.
Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature.
2002;
418
:
50-56
.
-
A.E.
Lang,
A.M.
Lozano.
Parkinson’s Disease. New England Journal of Medicine.
1998;
339
:
1044-1053
.
-
V.M.Y.
Lee,
R.
Daughenbaugh,
J.Q.
Trojanowski.
Microtubule stabilizing drugs for the treatment of Alzheimer’s disease. Neurobiology of Aging.
1994;
15
:
87-89
.
-
Y.-M.
Li,
M.
Xu,
M.-T.
Lai,
Q.
Huang,
J.L.
Castro,
J.
DiMuzio-Mower,
T.
Harrison,
C.
Lellis,
A.
Nadin,
J.G.
Neduvelil.
Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin 1. Nature.
2000;
405
:
689-694
.
-
X.
Liu,
F.
Li,
E.A.
Stubblefield,
B.
Blanchard,
T.L.
Richards,
G.A.
Larson,
Y.
He,
Q.
Huang,
A.-C.
Tan,
D.
Zhang.
Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell.
2012;
research22
:
321-332
.
-
K.
Lloyd,
L.
Davidson,
O.
Hornykiewicz.
The neurochemistry of Parkinson’s disease: effect of L-dopa therapy. Journal of Pharmacology and Experimental Therapeutics.
1975;
195
:
453-464
.
-
P.T.
Männistö,
S.
Kaakkola.
Rationale for Selective COMT Inhibitors as Adjuncts in the Drug Treatment of Parkinson’s Disease. Pharmacology & Toxicology.
1990;
66
:
317-323
.
-
L.
Mazzini,
K.
Mareschi,
I.
Ferrero,
E.
Vassallo,
G.
Oliveri,
R.
Boccaletti,
L.
Testa,
S.
Livigni,
F.
Fagioli.
Autologous mesenchymal stem cells: clinical applications in amyotrophic lateral sclerosis.. Neurological Research.
2006;
28
:
523-526
.
-
S.
Medvedev,
A.
Shevchenko,
S.
Zakian.
Induced pluripotent stem cells: problems and advantages when applying them in regenerative medicine. Acta Naturae (англоязычная версия) 2.
2010
.
-
R.G.
Miller,
J.
Mitchell,
M.
Lyon,
D.H.
Moore.
Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). The Cochrane Library.
2007
.
-
J.
Mitchell,
J.H.
Wokke,
G.D.
Borasio.
Recombinant human insulin-like growth factor I (rhIGF-I) for amyotrophic lateral sclerosis/motor neuron disease. The Cochrane Library.
2007
.
-
D.
Neary,
J.S.
Snowden,
L.
Gustafson,
U.
Passant,
D.
Stuss,
S.
Black,
M.
Freedman,
A.
Kertesz,
P.H.
Robert,
M.
Albert.
Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. eurology.
1998;
51
:
1546-1554
.
-
K.
Okita,
T.
Ichisaka,
S.
Yamanaka.
Generation of germline-competent induced pluripotent stem cells. Nature.
2007;
448
:
313-317
.
-
R.W.
Orrell,
R.J.M.
Lane,
M.
Ross.
Antioxidant treatment for amyotrophic lateral sclerosis/motor neuron disease. In Protocols (Wiley-Blackwell).
2005
.
-
Z.P.
Pang,
N.
Yang,
T.
Vierbuchen,
A.
Ostermeier,
D.R.
Fuentes,
T.Q.
Yang,
A.
Citri,
V.
Sebastiano,
S.
Marro,
T.C.
Südhof.
Induction of human neuronal cells by defined transcription factors. Nature.
2011
.
-
P.
Piccini,
D.J.
Brooks,
A.
Björklund,
R.N.
Gunn,
P.M.
Grasby,
O.
Rimoldi,
P.
Brundin,
P.
Hagell,
S.
Rehncrona,
H.
Widner.
Nat Neurosci 2. 1999;
:
1137-1140
.
-
M.
Prince,
G.-C.
Ali,
M.
Guerchet,
A.M.
Prina,
E.
Albanese,
Y.-T.
Wu.
Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s Research & Therapy.
2016;
8
.
-
L.
Puglielli,
R.E.
Tanzi,
D.M.
Kovacs.
Alzheimer’s disease: the cholesterol connection. Nature Neuroscience.
2003;
6
:
345-351
.
-
T.
Qu,
C.L.
Brannen,
H.M.
Kim,
K.
Sugaya.
Human neural stem cells improve cognitive function of aged brain. Neuroreport.
2001;
12
:
1127-1132
.
-
S.
Raitano,
L.
Ordovàs,
L.
De Muynck,
W.
Guo,
I.
Espuny- Camacho,
M.
Geraerts,
S.
Khurana,
K.
Vanuytsel,
Balazs I.
Tóth,
T.
Voets.
Restoration of Progranulin Expression Rescues Cortical Neuron Generation in an Induced Pluripotent Stem Cell Model of Frontotemporal Dementia. Stem Cell Reports.
2015;
4
:
16-24
.
-
E.
Ratnavalli,
C.
Brayne,
K.
Dawson,
J.R.
Hodges.
The prevalence of frontotemporal dementia. eurology.
2002;
58
:
1615-1621
.
-
D.E.
Redmond,
K.B.
Bjugstad,
Y.D.
Teng,
V.
Ourednik,
J.
Ourednik,
D.R.
Wakeman,
X.H.
Parsons,
R.
Gonzalez,
B.C.
Blanchard,
S.U.
Kim.
Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proceedings of the National Academy of Sciences.
2007;
104
:
12175-12180
.
-
E.D.
Roberson,
L.
Mucke.
100 Years and Counting: Prospects for Defeating Alzheimer’s Disease. cience.
2006;
314
:
781-784
.
-
J.V.
Sanchez-Mut,
H.
Heyn,
E.
Vidal,
S.
Moran,
S.
Sayols,
R.
Delgado-Morales,
M.D.
Schultz,
B.
Ansoleaga,
P.
Garcia- Esparcia,
M.
Pons-Espinal.
Human DNA methylomes of neurodegenerative diseases show common epigenomic patterns. Translational Psychiatry.
2016;
6
:
e718
.
-
D.
Schenk.
Hopes remain for an Alzheimer’s vaccine. Nature.
2004;
431
:
398-398
.
-
R.S.
Schwab.
Amantadine in the Treatment of Parkinson’s Disease. JAMA.
1969;
208
:
1168
.
-
M.
Suzuki,
C.N.
Svendsen.
Combining growth factor and stem cell therapy for amyotrophic lateral sclerosis. Trends in Neurosciences.
2008;
31
:
192-198
.
-
D.C.
Swinney,
J.
Anthony.
How were new medicines discovered?. Nature Reviews Drug Discovery.
2011;
10
:
507-519
.
-
Y.
Takagi,
J.
Takahashi,
H.
Saiki,
A.
Morizane,
T.
Hayashi,
Y.
Kishi,
H.
Fukuda,
Y.
Okamoto,
M.
Koyanagi,
M.
Ideguchi.
Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. Journal of Clinical Investigation.
2005;
115
:
102-109
.
-
B.
Uttara,
A.
Singh,
P.
Zamboni,
R.
Mahajan.
Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Current Neuropharmacology.
2009;
7
:
65-74
.
-
B.
Waldau,
A.K.
Shetty.
Behavior of neural stem cells in the Alzheimer brain. Cell Mol Life Sci.
2008;
65
:
2372-2384
.
-
Q.
Wang,
Y.
Matsumoto,
T.
Shindo,
K.
Miyake,
A.
Shindo,
M.
Kawanishi,
N.
Kawai,
T.
Tamiya,
S.
Nagao.
Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. The Journal of Medical Investigation.
2006;
53
:
61-69
.
-
S.
Weggen,
J.L.
Eriksen,
P.
Das,
S.A.
Sagi,
R.
Wang,
C.U.
Pietrzik,
K.A.
Findlay,
T.E.
Smith,
M.P.
Murphy,
T.
Bulter.
A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature.
2001;
414
:
212-216
.
-
M.L.
Weiss,
S.
Medicetty,
A.R.
Bledsoe,
R.S.
Rachakatla,
M.
Choi,
S.
Merchav,
Y.
Luo,
M.S.
Rao,
G.
Velagaleti,
D.
Troyer.
Human Umbilical Cord Matrix Stem Cells: Preliminary Characterization and Effect of Transplantation in a Rodent Model of Parkinson’s Disease. Stem Cells.
2006;
24
:
781-792
.
-
M.
Wernig,
A.
Meissner,
R.
Foreman,
T.
Brambrink,
M.
Ku,
K.
Hochedlinger,
B.E.
Bernstein,
R.
Jaenisch.
In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature.
2007;
448
:
318-324
.
-
P.J.
Whitehouse,
D.L.
Price,
A.W.
Clark,
J.T.
Coyle,
M.R.
DeLong.
Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Annals of Neurology.
1981;
10
:
122-126
.
-
H.
Wichterle,
I.
Lieberam,
J.A.
Porter,
T.M.
Jessell.
Directed Differentiation of Embryonic Stem Cells into Motor Neurons. Cell.
2002;
110
:
385-397
.
-
C.M.
Wischik,
P.C.
Edwards,
R.Y.
Lai,
M.
Roth,
C.R.
Harrington.
Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proceedings of the National Academy of Sciences.
1996;
93
:
11213-11218
.
-
U.
Wojda,
J.
Kuznicki.
Alzheimer’s disease modeling: ups, downs, and perspectives for human induced pluripotent stem cells. Journal of Alzheimer’s Disease.
2013;
34
:
563-588
.
-
Chris M.
Woodard,
Brian A.
Campos,
S.-H.
Kuo,
Melissa J.
Nirenberg,
Michael W.
Nestor,
M.
Zimmer,
E.V.
Mosharov,
D.
Sulzer,
H.
Zhou,
D.
Paull.
iPSC-Derived Dopamine Neurons Reveal Differences between Monozygotic Twins Discordant for Parkinson’s Disease. Cell Reports.
2014;
9
:
1173-1182
.
-
A.G.
Xuan,
D.H.
Long,
H.G.
Gu,
D.D.
Yang,
L.P.
Hong,
S.L.
Leng.
BDNF improves the effects of neural stem cells on the rat model of Alzheimer’s disease with unilateral lesion of fimbria-fornix. Neuroscience Letters.
2008;
440
:
331-335
.
-
S.
Yamanaka.
Induced Pluripotent Stem Cells: Past, Present, and Future. Cell Stem Cell.
2012;
10
:
678-684
.
-
M.B.H.
Youdim,
Y.S.
Bakhle.
Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. British Journal of Pharmacology.
2009;
147
:
S287-S296
.
-
Zoccolella.
Current and emerging treatments for amyotrophic lateral sclerosis. NDT.
2009;
577
.
Comments

Downloads
Article Details
Volume & Issue : Vol 3 No 07 (2016)
Page No.: 699-706
Published on: 2016-07-26
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 4630 times
- Download PDF downloaded - 1723 times
- View Article downloaded - 19 times
 Biomedpress
Biomedpress