Abstract
Objectives: This study aimed to investigate whether selecting euploid embryos by preimplantation genetic testing for aneuploidy (PGT-A) can improve the clinical outcomes in patients with advanced maternal age. Hence, it provides evidence about the role of PGT-A in the treatment for patients with advanced maternal age in Vietnam.
Methods: This is a retrospective cohort study, conducted at IVFMD, My Duc Hospital, Vietnam, from March 2017 to March 2019. There were 244 patients taking preimplantation genetic testing for aneuploidy (PGT-A group). Biopsy was performed at the blastocyst stage. On the day of biopsy, about 5-6 trophectoderm cells were collected and sent to analysis, while the remaining was individually vitrified to be used for embryo transfer to the patient. When patients had PGT-A, the clinician consulted and indicated the euploid embryo for frozen embryo transfer cycle. The ongoing pregnancy rate was compared with the group of patients who only performed blastocyst transfer (non-PGT-A group). Other outcomes, such as the average number of transferred embryos, clinical pregnancy rate, implantation rate, miscarriage rate and multiple pregnancy rate, were also compared between the two groups.
Results: In the total of 493 patients fulfilled the inclusion criteria, there were 244 patients in PGT-A group and 249 patients in non-PGT-A group. The patient characteristics of the two groups were similar (p > 0.05). A total of 816 blastocysts were biopsied and 315 (38.6%) of these were aneuploidy. The ongoing pregnancy rate of PGT-A group was significantly higher than non-PGT-A group (43.9% vs. 32.1%, p = 0.01). Moreover, mean number of transferred embryos and multiple pregnancy rate of PGT-A group was lower than non-PGT-A group (1.3 vs. 2, p < 0.001; 5.7% vs. 12%, p < 0.001, respectively).
Conclusions: In patients with advanced maternal age, the transfer of euploidy embryos selected by PGT-A improved the ongoing pregnancy rate and reduced the number of transferred embryos and multiple pregnancy rate. Therefore, this group of patients may benefit from PGT-A.
Introduction
In the modern society, women tend to get married and have children later than the previous generations. Therefore, the number of advanced maternal age (AMA) patients performing in-vitro fertilization (IVF) is increasing1. However, women fertility rate is inversely proportional to their age due to impairment of the ovarian reserve2 and increased abnormal oocytes3,4, which leads to the increase in embryonic aneuploidy rate. Aneuploidy is a common genetic abnormality in humans. Studies showed that the majority of embryo aneuploidy had a maternal origin5,6. This rate is higher as a woman gets older, and about 50% of the embryos from IVF treatment are aneuploidy7,8,9. Aneuploidy is the main reason causing implantation failure, early miscarriage, and prolonged time to pregnancy in IVF4. Most of the aneuploidy occurs due to mitotic and meiotic error arisen in the preimplantation embryo stage10. In women over 35 years old, aneuploidy embryos may result in miscarriage, including natural pregnancy and IVF treatment cycles9. Preimplantation genetic testing to detect aneuploidy of embryo is increasingly popular all over the world. Preimplantation genetic testing for aneuploidy (PGT-A) biopsy approache includes biopsy of polar bodies from the oocyte4,11, biopsy of blastomeres from cleavage-stage embryo, or trophectoderm (TE) cells from blastocyst embryo12,13. However, the biopsy of polar bodies only contains DNA of oocytes (maternal contributions) and does not represent the DNA in the embryonic status. There is evidence showing that the biopsy of blastomere is not only an invasive technique affecting the development and embryonic implantation potential12,14,15, but also logistically difficult and costly16. Nowadays, biopsy of TE cells is currently the most widely used approach accounting for IVF centers17,18,19. TE biopsy intends to remove only 5-6 cells from the trophectoderm. Blastocyst of biopsy is the least invasive technique and does not affect the embryonic development and implantation potential12.
Multiple pregnancies are the primary concern of IVF centers. During the last decades, the common practice of transferring more than one embryo into the uterus was used to increase the clinical pregnancy rate. Although many centers have reduced the number of transferred embryos to decrease multiple pregnancy rate, the twinning rate remains high because double embryo transfer is often performed in their treatment cycle. Many studies have reported that twin pregnancy affects reproductive health and the cost of care for the newborns20,21,22. Therefore, the choice of single embryo transfer with high implantation potential is the goal of most of IVF centers.
PGT-A is the technique to select euploid embryos with the best implantation potential. This technique has been applied to treat patients with an increased risk of having aneuploid embryos, such as those with advanced maternal age1,23, repeated implantation failure24, and recurrent miscarriage25,26,27. Up to date, PGT-A studies remain limited and have not been reported for the clinical outcomes in patients with advanced maternal age in Vietnam. Therefore, more evidence is needed for aneuploidy testing in this group of patients.
The purpose of our study was to assess the clinical outcomes following blastocyst biopsy and frozen euploid embryo transfer by using (PGT-A) for AMA patients in Vietnam.
MATERIALS - METHODS
Patient selection and study design
This is a retrospective cohort study. The data were obtained from 493 women (35 to 45 years old) from March 2017 to March 2019 at IVFMD (My Duc Hospital, Vietnam). This study was approved by the Reproductive Health and the Ethical Board of My Duc Hospital. The individual information was coded to ensure patient privacy. Exclusion criteria were as follows: in-vitro maturation (IVM) cycles, IUI change to ICSI cycles, patients with repeated implantation failure, recurrent miscarriage, and uterine abnormal. Patients were divided into two groups: PGT-A (244 patients) and non-PGT-A (249 patients).
Sperm preparation
Both frozen and fresh semen samples were prepared by discontinuous density gradient centrifugation. This method helped to separate motile spermatozoa from seminal plasma. Two layers were formed with a 40% density top layer and an 80% density lower layer. The centrifugation helped motile spermatozoa swim through the gradient materials to form a soft pellet at the bottom of the tube. After that, the soft pellet was collected and washed to be used for ICSI28.
Ovarian stimulation and Oocyte Retrieval
The ovarian stimulation was carried out with a GnRH Antagonist protocol and ovulation is triggered by hCG or agonist injection. Follicle development was followed by ultrasound, and checked for estradiol and progesterone levels. Oocyte retrieval was done at 36 hours after hCG or agonist injection when at least two follicles reached 14 mm. Upon retrieval, oocyte cumulus complexes were rinsed and cultured in Global Total for Fertilization medium (Life Global – Canada), supplemented with bicarbonate buffer, lactate and pyruvate, at 37oC, 6% CO2 and 5% O2 in the incubator. After that, the denudation of cumulus cells surrounding the oocytes was performed by using hyaluronidase (Origio- Denmark) and mechanical pipetting. Only matured oocytes (Metaphase II) were injected.
Embryo culture
Intracytoplasmic sperm injection (ICSI) was performed on the matured oocytes approximately 39 to 41 hours after hCG or agonist injection. Following the fertilization, embryos were placed in the Benchtop (G310, K-system, Denmark) incubator at 37oC with CO2 and 5% O2.
Fertilization and cleavage-stage embryo evaluation
Fertilization analysis was assessed at 16-18 hours after ICSI. On day 3, the cleavage-stage embryos were evaluated base on the number of blastomeres, the size of blastomeres and the embryo fragmentation according to embryo assessment guidelines at IVFMD.
Blastocyst evaluation
The blastocyst was evaluated at 112 to 116 hours (on day 5) after ICSI, according to embryo assessment guidelines at IVFMD (based on Alpha Scoring System, 2011)29. Embryo quality was evaluated by morphology under the inverted microscope (Zeizz, Germany). The evaluation process was based on the degree of expansion, the number of cells and cell compaction of the inner cell mass (ICM), and the trophectoderm of the blastocyst. The high-quality embryos (grade 1 and grade 2) were classified with blastocoel filling greater than half the volume of the embryo, the ICM with tightly packed/ loosely grouped cells and the trophectoderm with cells forming cohesive epithelium or few cells forming lose epithelium (Figure 1a, b). The poor-quality embryos (grade 3) were classified with any other expansion degrees, the ICM with very few cells or degradation, and the trophectoderm with very few large cells or degradation (Figure 1c). The high-quality embryos were prioritized for biopsy, vitrification, or transferring while the poor-quality embryos were not prioritized for biopsy or freeze.
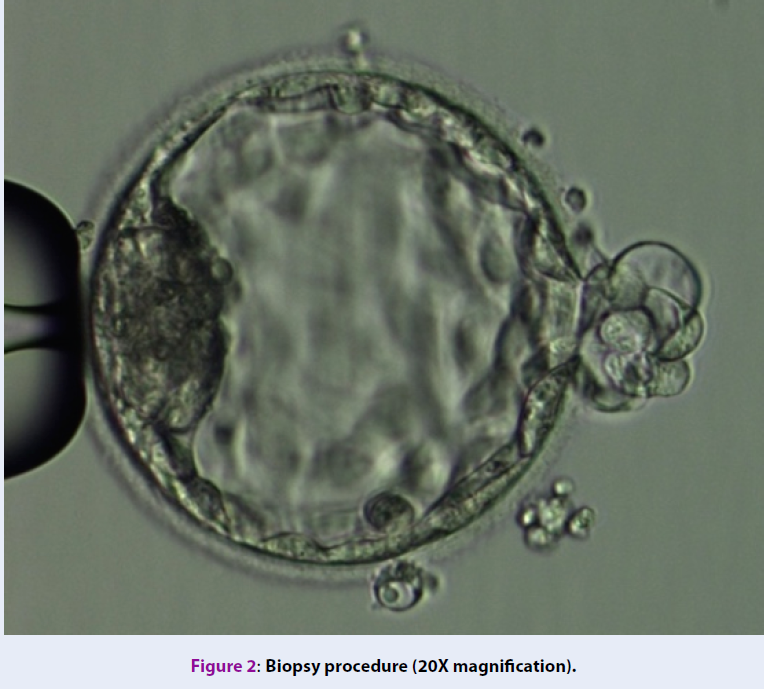
Biopsy of blastocyst
The selection of embryos for biopsy follows the consensus at IVFMD (based on Alpha Scoring System, 2011)29. Biopsy samples contained 5-6 TE cells (Figure 2). The TE cells were washed in phosphate-buffered saline solution (PBS-Merk, Germany) and then stored in the microcentrifuge tubes containing 2 μl PBS and were genetically analyzed at the genetic analysis laboratory (Tan Son Nhat Hospital, 2 Pho Quang Street, 2 Ward, Tan Binh District, Ho Chi Minh city).
Embryo freezing, warming and transfer
After blastocyst biopsy, the embryo was frozen using Kuwayama protocol30 with Vitrification kit (Cryotech, Japan), and was individually loaded onto carrier tool based on IVFMD’s laboratory routine standardization. When genetic testing results were available, only the euploid embryo was chosen for transfer in the first of the frozen embryo transfer (FET) cycle. The embryo was thawed and morphology was assessed, hatching was assisted, and embryo was transferred with a specialized catheter under ultrasonographic guidance. After the thawing procedure, the thawed embryo morphology was assessed before it was transferred to patients. If the embryo showed degradation cells, it depended on the number of degrading cells; we decided to transfer this embryo or announced the patient to thaw another embryo. In this study, all thawed embryo could be transferred to the patient without having to thaw another. In FET cycle, the patients were transferred with one or two embryos, which is dependent on the patient’s decision.
Clinical outcome assessment
The primary outcome was the ongoing pregnancy rate in the first frozen embryo transfer cycle of both groups. The ongoing pregnancy rate was defined as the percentage of embryos transferred that produced an implanted embryo and had the embryocardia under ultrasound up to week 12 of pregnancy31. The ongoing pregnancy rate was compared with the group of patients who only performed blastocyst transfer (non-PGT-A group). Other outcomes, such as the average number of transferred embryos, clinical pregnancy rate, implantation rate, miscarriage rate and multiple pregnancy rate were also compared between the two groups.
Statistical analysis
The baseline characteristics of the study population were described by descriptive statistics. For the study outcomes, histogram and Shapiro test were used to check for normal distribution and continuous variables. If the variables have a normal distribution, the data were presented as mean, standard deviation and compared by a Student t-test. If the variables were not normally distributed, the data were presented as median and quartile, non-parametric tests were used to check for differences between groups. For the category, we used percentages between the two branches and compared them with Pearson’s chi-square test or Fisher’s exact test if they were appropriate. All tests were two-tailed tests, p-values of less than 0.05 (p<0.05) were considered as statistically significant. Values were expressed as mean ± standard deviation (SD).
| PGT-A (N=244) | non-PGT-A (N=249) | P-value | |
|---|---|---|---|
| The baseline characteristics | |||
| Age (years) | 38.1 ± 2.7 | 38.4 ± 0.6 | 0.128 |
| BMI (kg/m2) | 21.7 ± 2.3 | 21.8 ± 2.6 | 0.657 |
| AMH level (ng/ml) | 4.1 ± 3.0 | 4.0 ± 3.1 | 0.593 |
| Duration of infertility (years) | 6.0 ± 4.2 | 6.5 ± 4.4 | 0.168 |
| Estradiol level on hCG day (pmol/L) | 8985.3 ± 10861.7 | 7126.4 ± 6253.9 | 0.03 |
| Progesterone level on hCG day (pmol/L) | 1.8 ± 4.1 | 1.6 ± 3.5 | 0.633 |
| Total days of stimulation (days) | 8.9 ± 1.3 | 15.8 ± 113.8 | 0.34 |
| Total of FSH dose (IU) | 2476.6 ± 533.6 | 2379.6 ± 692.6 | 0.088 |
RESULTS
The baseline characteristics and ovarian stimulation results were showed in Table 1. Overall baseline characteristics and ovarian stimulation data including age, BMI, AMH level, duration of infertility, days of stimulation and total FSH were not significantly different between the two groups (p > 0.05). Since the primary patient's characteristics were similar between two groups (Table 1), the consequent outcomes would be more objectivity and reliability.
The total of 493 patients underwent this study. There was a total of 7614 retrieved oocytes with 6258 mature oocytes, which were performed ICSI. The number of fertilized oocytes were 5344, with fertilization rate reached 85.4%. A total of 3017 blastocysts with blastocyst rate reached 48.1%. The detailed parameters of each group were showed in Table 2.
| PGT-A (N=244) | non-PGT-A (N=249) | P - value | |
|---|---|---|---|
| Number of retrieved oocytes | 16.0 ± 8.3 | 14.9 ± 4.5 | 0.061 |
| Number of MII oocytes | 13.4 ± 7.1 | 12.0 ± 4.1 | 0.008 |
| Number of fertilized oocytes | 11.8 ± 6.1 | 9.9 ± 3.7 | < 0.001 |
| 2PN rate | 78.1 ± 20.0 | 74.3 ± 19.1 | 0.03 |
| Blastocyst rate | 48.1 ± 24.1 | 55.5 ± 20.3 | < 0.001 |
| Number of blastocyst | 6.0 ± 4.1 | 6.6 ± 3.2 | 0.108 |
| Number of good quality blastocyst | 3.0 ± 2.8 | 4.2 ± 2.8 | < 0.001 |
| Number of blastocyst for PGT-A | 3.4 ± 1.9 | - | - |
PGT-A group showed a similar average number of retrieved oocytes (16.0 ± 8.3 vs. 14.9 ± 4.5 oocytes, p = 0.61) as compared to non-PGT-A group. PGT-A group showed higher average number of MII oocytes (13.4 ± 7.1 vs. 12.0 ± 4.1 oocytes, p = 0.008), fertilized oocytes (11.8 ± 6.1 vs. 9.9 ± 3.7 oocytes, p < 0.001) and 2PN rate (78.1 ± 20.0 vs. 74.3 ± 19.1%, p = 0.03) compared to non-PGT-A group. However, the blastocyst rate and number of good quality blastocyst embryos in the PGT-A group were lower than the non-PGT-A group (48.1 ± 24.1 vs. 55.5 ± 20.3%, p < 0.001; 3.0 ± 2.8 vs. 4.2 ± 2.8 embryos, p < 0.001, respectively), while two groups showed no statistically significant differences in the average number of blastocyst embryos (6.0 ± 4.1 vs. 6.6 ± 3.2 embryos, p = 0.108).
In PGT-A group, the mean of biopsy blastocyst was 3.4 ± 1.9 blastocyst embryos. From all patients, a total of 816 blastocyst embryos were biopsied and genetically analyzed. The euploid embryos had the highest rate (59.9%), followed by aneuploid embryo rate (38.6%), and finally mosaic embryo rate (1.5%). In the group of the aneuploid embryo (489 embryos), there were 73 complex embryos (23.2%), 92 trisomy embryos (29.2%), 100 monosomy embryos (31.7%) and 50 structurally abnormal embryos (15.9%). These results showed that the percentage of the aneuploid embryo was higher in AMA patients, approximately accounting for half of biopsied embryo (Table 3 ).
| PGT-A (N=816) | |
|---|---|
| Euploidy - n (%) | 489 (59.9) |
| Mosaic - n (%) | 12 (1.5) |
| Aneuploidy - n (%) | 315 (38.6) |
| Complexs | 73 (23.2) |
| Trisomy | 92 (29.2) |
| Monosomy | 100 (31.7) |
| Structural abnormality | 50 (15.9) |
| Parameters | PGT-A (N=244) | non-PGT-A (N=249) | P - value |
|---|---|---|---|
| Number of embryo transferred | 1.3 ± 0.4 | 2.0 ± 0.4 | < 0.001 |
| -hCG positive rate | 61.5% | 43.0% | < 0.001 |
| Clinical pregnancy rate | 54.9% | 38.6% | < 0.001 |
| Multiple pregnancy rate | 5.7% | 12.0% | < 0.001 |
| Implantation rate | 49.3% | 40.2% | < 0.001 |
| Miscarriage rate | 11.1% | 6.4% | 0.096 |
| Ongoing pregnancy rate | 43.9% | 32.1% | 0.01 |
| Live birth rate | 38.5% | 30.9% | 0.093 |
| Birth Weight (gram) – (sd) | 2918 ± 538 | 3027 ± 621 | 0.082 |
The ongoing pregnancy rate of PGT-A group was significantly higher than the non-PGTA group (43.9% vs. 32.1%, p = 0.01). The PGT-A group also showed higher average number of the -hCG positive rate, implantation rate and clinical pregnancy rate than non-PGT-A group (61.5% vs. 43.0%, 49.3% vs. 40.2%, 54.49% vs. 38.6%, p < 0.001, respectively). In contrast, the mean number of embryos transferred and the multiple pregnancy rate in the PGT-A group was significantly lower than the non-PGT-A group (1.3 vs. 2.0 embryos, 5.7% vs. 12.0%, p < 0.001, respectively). There was no statistically significant difference in the miscarriage rate between the two groups (11.1% vs. 6.4%, p = 0.096). The live birth rate of PGT-A group was higher than non-PGT-A but not statistically different (38.5% vs. 30.9%, p = 0.093). The result showed that the birth weight was similar between the two groups (2918 g vs. 3027 g, p= 0.082) (Table 4 ).
DISCUSSION
The goal of the PGT-A was to select single euploid embryo to transfer to IVF patients to increase the chance of pregnancy and having a healthy baby. The aneuploidy rate was increased in patients, who took IVF treatment at advanced maternal age9,32,33. The probability of successful development and implantation at the early stage of the embryo depends on the genetic status. Subsequent errors in the genetic of the embryos can lead to embryonic mortality. Aneuploidy (monosomy and trisomy) is the most common type of abnormal chromosome in humans. The aneuploid embryos could not develop in the uterus after they were transferred; therefore, the frozen embryo transfer cycle using these aneuploid embryos would significantly decrease the pregnancy rate of the patients.
For this reason, euploidy embryo selection for transfer can improve implantation rate as well as the ongoing pregnancy rate of advanced maternal age. The study of Minasi (2016) showed that mean maternal age in the euploidy was younger than aneuploidy group34. Moreover, this research was that emphasized the aneuploidy rate rises approximately 10% per year of female age. Therefore, it is necessary to use appropriate technique for identifying euploidy without reducing the embryo implantation potential.
Our research compared the clinical outcomes between AMA patients with or without transferring the euploid embryo selected by PGT-A. The ongoing pregnancy rate of PGT-A group was significantly higher than non-PGT-A group (43.9% vs. 32.1%, p=0.01). Moreover, the mean number of embryos transferred and the multiple pregnancy rate of the PGT-A group was lower than non-PGT-A group (1.3 vs. 2.0 embryos, p<0.001; 5.7% vs. 12%, p<0.001, respectively).
A multicenter, randomized clinical trial by Rubio et al., 2017 assessed clinical outcomes in advanced maternal age between 38 and 41 years with euploidy transferred showed that the miscarriage rate of PGT-A group exhibited significantly lower than the control group (2.7% vs. 39.0%, p < 0.001). The pregnancy rate at the first transfer attempt was higher (52.9% vs. 24.2%, p < 0.001), lower the number of transferred embryos (1.3 vs. 1.8, p < 0.0001) and the time to achieve a live birth of PGT-A group were also lower compared to the non PGT-A group (7.7 weeks vs. 14.9 weeks)1.
Similar to our results, the study of Schoolcraft vs. Katz-Jaffe (2013) determined that the ongoing pregnancy rate of the euploid blastocyst transferred group was higher than the control group (60.0% vs. 43.8%, p< 0.05)6. The report of Harton (2013) indicated that the implantation rate and the ongoing pregnancy rate were constant in patients of 35 to 42 years old, who performed PGT-A and single euploid embryo transfer35. Recently, a publication of Taiwanese researchers showed that the AMA patients with ICSI/ PGT-A and the euploidy transfer had significantly higher live birth rate than non-PGT-A group23.
Chang et al. (2019) compared the women who underwent PGT-A with a single euploid frozen embryo transfer group versus the women who underwent a multiple unscreened embryo transfer of fresh embryo cycles in women ≥ 43 years. This study outcomes showed that the study group had significantly higher implantation rate (56.9% vs. 13.8%, OR 8.9 [95% CI 4.9-16.3]) and ongoing pregnancy rate (50.0% vs. 6.9%, OR 14.3 [95% CI 7.3-28.0]), and lower early miscarriage pregnancy rate (18.3% vs. 46.2%, OR 0.25, [95% CI 0.11-0.57]) and clinical miscarriage pregnancy rate (12.1% vs. 50.0%, OR 0.16 [95% CI 0.06-0.47]), compared to control group36.
Another study of Verpoest W (2018) demonstrated that live birth rate between PGT-A and non-PGT-A groups were similar (24%; 95% CI: -7.60- 9.18%), while the PGT-A group have lower miscarriage rate than the control group. However, the sample size of PGT-A group was small, thus, the number of euploidy transferred in PGT-A group was higher (41% double embryo transfer) leading to high multiple pregnancy rate and miscarriage rate3.
Besides, Shelby A. Neal (2018) compared the cost- effective and clinical outcomes of patients with or without PGT-A37. Comparing the cost-effective outcomes on patient’s age, excluded patients who have only one blastocyst, the patients > 37 years of age saved more treatment cost than patients < 35 years of age. Furthermore, this study showed that the cumulative live births of PGT-A and control groups were identical but PGT-A reduced the time in treatments up to four months, and decreased the risk of embryo transfer failure and pregnancy loss37.
The limitation of our study is that it was a retrospective cohort study, in which we did not actively select samples for the study. We collected data on treated outcomes of the patient in IVFMD, My Duc Hospital, Vietnam from March 2017 to March 2019.
CONCLUSIONS
The transfer of euploid embryo improved the clinical outcomes of advanced maternal age. Specifically, our study showed that the euploid embryo selection by PGT-A increased clinical pregnancy rate, implantation rate, ongoing pregnancy rate and decreased multiple pregnancy rate due to the reduced number of embryo transfers. Therefore, PGT-A can be consulted to women with advanced maternal age, who carried out IVF at My Duc Hospital. However, this was a retrospective study, a better well-designed study with a more considerable sample size needs to be considered in the future.
ABBREVIATIONS
PGT-A: Preimplantation Genetic Testing- Aneuploidy
IVF: In- vitro fertilization
ICSI: Intracytoplasmic sperm injection
BMI: Body mass index
AMH: Antimuller hormone
FSH: Follical stimulating hormone
IVM: In-vitro Maturation
IUI: Intrauterine insemination
CONFLICT OF INTEREST
The authors declare that they have not conflicted of interests.
AUTHORS’ CONTRIBUTIONS
LTBP wrote the manuscript. VNT and LHA revised the manuscript. DQV planned and designed the experiments. PTQ collected and analysed the data. NTTH supervised the study and finalized the manuscript. All authors read and confirmed the publication of the article.
ACKNOWLEDGMENTS
This study was performed at IVFMD-My Duc Hospital and IVFMD PN-My Duc Phu Nhuan Hospital. The authors acknowledge Directors to support the data and devices for this study. We are also thankful to our colleague from IVFMD PN who positively assisted in completing this study.
References
-
Rubio
C.,
Bellver
J.,
Rodrigo
L.,
Castillón
G.,
Guillén
A.,
Vidal
C.,
In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertility and Sterility.
2017;
107
(5)
:
1122-9
.
View Article PubMed Google Scholar -
Hassold
T.,
Hunt
P.,
Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Current Opinion in Pediatrics.
2009;
21
(6)
:
703-8
.
View Article PubMed Google Scholar -
Fragouli
E.,
Alfarawati
S.,
Spath
K.,
Jaroudi
S.,
Sarasa
J.,
Enciso
M.,
The origin and impact of embryonic aneuploidy. Human Genetics.
2013;
132
(9)
:
1001-13
.
View Article PubMed Google Scholar -
Verpoest
W.,
Staessen
C.,
Bossuyt
P.M.,
Goossens
V.,
Altarescu
G.,
Bonduelle
M.,
Preimplantation genetic testing for aneuploidy by microarray analysis of polar bodies in advanced maternal age: a randomized clinical trial. Human Reproduction (Oxford, England).
2018;
33
(9)
:
1767-76
.
View Article PubMed Google Scholar -
Webster
A.,
Schuh
M.,
Mechanisms of Aneuploidy in Human Eggs. Trends in Cell Biology.
2017;
27
(1)
:
55-68
.
View Article PubMed Google Scholar -
McCallie
B.R.,
Parks
J.C.,
Trahan
G.D.,
Jones
K.L.,
Coate
B.D.,
Griffin
D.K.,
Compromised global embryonic transcriptome associated with advanced maternal age. Journal of Assisted Reproduction and Genetics.
2019;
36
(5)
:
915-24
.
View Article PubMed Google Scholar -
Kuliev
A.,
Cieslak
J.,
Ilkevitch
Y.,
Verlinsky
Y.,
Chromosomal abnormalities in a series of 6,733 human oocytes in preimplantation diagnosis for age-related aneuploidies. Reproductive Biomedicine Online.
2003;
6
(1)
:
54-9
.
View Article PubMed Google Scholar -
Rabinowitz
M.,
Ryan
A.,
Gemelos
G.,
Hill
M.,
Baner
J.,
Cinnioglu
C.,
Origins and rates of aneuploidy in human blastomeres. Fertility and Sterility.
2012;
97
(2)
:
395-401
.
View Article PubMed Google Scholar -
Franasiak
J.M.,
Forman
E.J.,
Hong
K.H.,
Werner
M.D.,
Upham
K.M.,
Treff
N.R.,
The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertility and Sterility.
2014;
101
(3)
.
View Article PubMed Google Scholar -
Sermon
K.,
Novel technologies emerging for preimplantation genetic diagnosis and preimplantation genetic testing for aneuploidy. Expert Review of Molecular Diagnostics.
2017;
17
(1)
:
71-82
.
View Article PubMed Google Scholar -
Geraedts
J.,
Collins
J.,
Gianaroli
L.,
Goossens
V.,
Handyside
A.,
Harper
J.,
What next for preimplantation genetic screening? A polar body approach!. Human Reproduction (Oxford, England).
2010;
25
(3)
:
575-7
.
View Article PubMed Google Scholar -
Cimadomo
D.,
Capalbo
A.,
Ubaldi
F.M.,
Scarica
C.,
Palagiano
A.,
Canipari
R.,
The Impact of Biopsy on Human Embryo Developmental Potential during Preimplantation Genetic Diagnosis. Biomed Res Int.
2016;
2016
.
View Article Google Scholar -
Homer
H.A.,
Preimplantation genetic testing for aneuploidy (PGT-A): the biology, the technology and the clinical outcomes. Australian and New Zealand Journal of Obstetrics and Gynaecology.
2019;
59
(2)
:
317-24
.
View Article PubMed Google Scholar -
Scott
R.T.,
Upham
K.M.,
Forman
E.J.,
Zhao
T.,
Treff
N.R.,
Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertility and Sterility.
2013;
100
(3)
:
624-30
.
View Article PubMed Google Scholar -
Scott
R.T.,
Upham
K.M.,
Forman
E.J.,
Hong
K.H.,
Scott
K.L.,
Taylor
D.,
Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertility and Sterility.
2013;
100
(3)
:
697-703
.
View Article PubMed Google Scholar -
Griffin
D.K.,
Ogur
C.,
Chromosomal analysis in IVF: just how useful is it?. Reproduction (Cambridge, England).
2018;
156
(1)
:
29-50
.
View Article PubMed Google Scholar -
He
H.,
Jing
S.,
Lu
C.F.,
Tan
Y.Q.,
Luo
K.L.,
Zhang
S.P.,
Neonatal outcomes of live births after blastocyst biopsy in preimplantation genetic testing cycles: a follow-up of 1,721 children. Fertility and Sterility.
2019;
112
(1)
:
82-8
.
View Article PubMed Google Scholar -
Victor
A.R.,
Griffin
D.K.,
Brake
A.J.,
Tyndall
J.C.,
Murphy
A.E.,
Lepkowsky
L.T.,
Assessment of aneuploidy concordance between clinical trophectoderm biopsy and blastocyst. Human Reproduction (Oxford, England).
2019;
34
(1)
:
181-92
.
View Article PubMed Google Scholar -
Fragouli
E.,
Alfarawati
S.,
Spath
K.,
Babariya
D.,
Tarozzi
N.,
Borini
A.,
Analysis of implantation and ongoing pregnancy rates following the transfer of mosaic diploid-aneuploid blastocysts. Human Genetics.
2017;
136
(7)
:
805-19
.
View Article PubMed Google Scholar -
Lemos
E.V.,
Zhang
D.,
Van Voorhis
B.J.,
Hu
X.H.,
Healthcare expenses associated with multiple vs singleton pregnancies in the United States. American Journal of Obstetrics and Gynecology.
2013;
209
(6)
.
View Article PubMed Google Scholar -
Pinborg
A.,
Loft
A.,
Schmidt
L.,
Andersen
A.N.,
Morbidity in a Danish national cohort of 472 IVF/ICSI twins, 1132 non-IVF/ICSI twins and 634 IVF/ICSI singletons: health-related and social implications for the children and their families. Human Reproduction (Oxford, England).
2003;
18
(6)
:
1234-43
.
View Article PubMed Google Scholar -
Sazonova
A.,
Källen
K.,
Thurin-Kjellberg
A.,
Wennerholm
U.B.,
Bergh
C.,
Neonatal and maternal outcomes comparing women undergoing two in vitro fertilization (IVF) singleton pregnancies and women undergoing one IVF twin pregnancy. Fertility and Sterility.
2013;
99
(3)
:
731-7
.
View Article PubMed Google Scholar -
Lee
C.I.,
Wu
C.H.,
Pai
Y.P.,
Chang
Y.J.,
Chen
C.I.,
Lee
T.H.,
Performance of preimplantation genetic testing for aneuploidy in IVF cycles for patients with advanced maternal age, repeat implantation failure, and idiopathic recurrent miscarriage. Taiwanese Journal of Obstetrics {&}amp; Gynecology.
2019;
58
(2)
:
239-43
.
View Article PubMed Google Scholar -
Greco
E.,
Bono
S.,
Ruberti
A.,
Lobascio
A.M.,
Greco
P.,
Biricik
A.,
Comparative genomic hybridization selection of blastocysts for repeated Implantation Failure Treatment: A Pilot Study. Biomed Res Int.
2014;
2014
.
View Article Google Scholar -
Murugappan
G.,
Ohno
M.S.,
Lathi
R.B.,
Cost-effectiveness analysis of preimplantation genetic screening and in vitro fertilization versus expectant management in patients with unexplained recurrent pregnancy loss. Fertility and Sterility.
2015;
103
(5)
:
1215-20
.
View Article PubMed Google Scholar -
Hodes-Wertz
B.,
Grifo
J.,
Ghadir
S.,
Kaplan
B.,
Laskin
C.A.,
Glassner
M.,
Idiopathic recurrent miscarriage is caused mostly by aneuploid embryos. Fertility and Sterility.
2012;
98
(3)
:
675-80
.
View Article PubMed Google Scholar -
El Hachem
H.,
Crepaux
V.,
May-Panloup
P.,
Descamps
P.,
Legendre
G.,
Bouet
P.E.,
Recurrent pregnancy loss: current perspectives. International Journal of Women{&}{#}x0027;s Health.
2017;
9
:
331-45
.
View Article PubMed Google Scholar -
Organization WH. Examination and processing of human semen. World Health [Internet]. 2010;Edition, F(10):286. Available from: http://whqlibdoc.who.int/publications/2010/9789241547789_eng.pdf.
.
-
Scientists In Reproductive Medicine
ALPHA,
Special Interest Group Embryology
ESHRE,
Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reproductive Biomedicine Online.
2011;
22
(6)
:
632-46
.
View Article PubMed Google Scholar -
Kuwayama
M.,
Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology.
2007;
67
(1)
:
73-80
.
View Article PubMed Google Scholar -
Yi
Y.,
Lu
G.,
Ouyang
Y.,
lin
G.,
Gong
F.,
Li
X.,
A logistic model to predict early pregnancy loss following in vitro fertilization based on 2601 infertility patients. Reproductive Biology and Endocrinology.
2016;
14
(1)
:
15
.
View Article PubMed Google Scholar -
Liu
J.,
Wang
W.,
Sun
X.,
Liu
L.,
Jin
H.,
Li
M.,
DNA microarray reveals that high proportions of human blastocysts from women of advanced maternal age are aneuploid and mosaic. Biology of Reproduction.
2012;
87
(6)
:
148
.
View Article PubMed Google Scholar -
Fragouli
E.,
Alfarawati
S.,
Daphnis
D.D.,
Goodall
N.N.,
Mania
A.,
Griffiths
T.,
Cytogenetic analysis of human blastocysts with the use of FISH, CGH and aCGH: scientific data and technical evaluation. Human Reproduction (Oxford, England).
2011;
26
(2)
:
480-90
.
View Article PubMed Google Scholar -
Minasi
M.G.,
Colasante
A.,
Riccio
T.,
Ruberti
A.,
Casciani
V.,
Scarselli
F.,
Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Human Reproduction (Oxford, England).
2016;
31
(10)
:
2245-54
.
View Article PubMed Google Scholar -
Harton
G.L.,
Munné
S.,
Surrey
M.,
Grifo
J.,
Kaplan
B.,
McCulloh
D.H.,
Practitioners Group
PGD,
Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertility and Sterility.
2013;
100
(6)
:
1695-703
.
View Article PubMed Google Scholar -
Chang
S.,
Nazem
T.G.,
Sekhon
L.H.,
Mukherjee
T.,
Lee
J.A.,
Copperman
A.B.,
Transfer of a single genetically screened embryo in women >43 results in significantly higher pregnancy rates and lower pregnancy loss rates than transfer of multiple unscreened embryos. Fertility and Sterility.
2019;
111
(4)
:
e35
.
View Article Google Scholar -
Neal
S.A.,
Morin
S.J.,
Franasiak
J.M.,
Goodman
L.R.,
Juneau
C.R.,
Forman
E.J.,
Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertility and Sterility.
2018;
110
(5)
:
896-904
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 12 (2019)
Page No.: 3541-3549
Published on: 2019-12-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 9858 times
- Download PDF downloaded - 2424 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress



