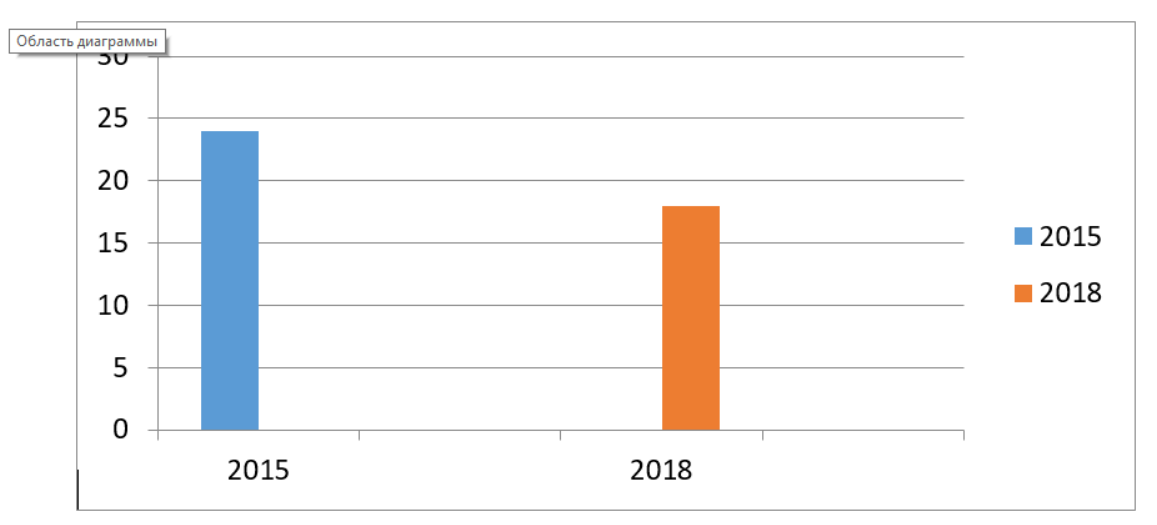Abstract
Introduction: This study addresses and evaluates the decrease of antibiotic resistance after introduction of a proposed prevention plan and control complex in the intensive care unit (ICU).
Methods: Data from 1,111 bacteriological analyses, taken from patients who received treatment in the ICU of Ternopil University Hospital from January to August 2015 (group 1) and the same period of 2018 (group II), were included in the study. The complex included measures for the prevention of antibiotic resistance spread and for rational antibiotic use.
Results: We found that resistance to imipenem changed more than other antibacterial drugs, increased by 60% (р ≤ 0.05), which was conditioned predominantly by Pseudomonas aeruginosa isolates for 100%. A decrease in 39% of polyresistant clinical isolates of Klebsiella pneumoniae in patients of groups I and II showed important prognostic value.
Conclusion: A complex of the proposed measures included the division of patients in blocks according to the risk of infectious complications, control of antibiotics administration, adherence to sanitary norms by ICU staff, use of sodium hypochlorite resulting in decrease of pathogenic isolates, and level of antibiotic resistance to specific groups of antibacterial drugs.
Introduction
Antibiotic resistance of microflora is currently not only a medical problem but also a significant social and economic issue1. Antibiotic-resistant bacteria could become a cause of death of 10 billion people a year by 2050, according to the predictions of experts2, 3; this would surpass mortality from oncologic diseases and diabetes mellitus together. Taking into account the speed of formation and spread of antibiotic resistance, there is a dire need for new generation antibiotics; however, this is a long-stretch process4, 5. Different strains of pathogens have known resistance to antibiotics, including multi-drug resistant strains6. Due to this, several international policies, including the European strategic action plan on antibiotic resistance and the Global action plan on antimicrobial resistance announced by the World Health Organization (WHO), have addressed the issues of antimicrobial resistance.
One of the critical needs is to decrease of risk of formation and spread of nosocomial strains of microorganisms which have resistance to antimicrobials. Steps to address this are the development and implementation of an effective program of infection control in hospitals7.
Materials — Methods
Analysis of 1,111 bacteriological investigations from patients, treated in the intensive care unit (ICU) of the Ternopil University clinics during the period of January to August 2015 (group I) and during the same period of 2018 (group II), were included in the study. The local Ethics Committee approved the conduction of the study after revision of the study protocol. The complex included measures for the prevention of antibiotic resistance spread and for rational antibiotic use. Patients were divided into blocks according to the probability of infectious complications. Block A consisted of patients with purulent infections or high risk of infectious complications. Block B consisted of post-operative patients, who require intensive care and have a low risk of infectious complications. Block C included patients after cardiac surgery with the lowest risk of infectious complications.
The principle of "cocoon" was maintained in each block: individual mechanical ventilation device, cardiovascular monitor, suction unit, stethoscope, blood pressure device, and antiseptics for personnel who were in contact with the patient. One nurse worked with a maximum of 2 patients due to lack of personnel. The strict control of hands and medical cloth hygiene were observed (e.g. daily change of clothes and restriction to move to different blocks in the same cloths), as were prevention of catheter-associated infections of the blood and urinary tract, prevention of ventilator-associated infections of the respiratory tract, and constant education among the staff of all surgical departments.
We controlled the administration of antibiotics according to the guidelines and recommendations and avoided causeless preventive administration of antibiotics. These steps led to the decrease of antibiotic administration in the Departments of Minimally Invasive Surgery and Orthopedics by 3-fold. Patients with the purulent process, especially with defined antibiotic resistance, underwent electrochemical detoxication with the administration of sodium hypochlorite solution in a dose of 600 mg/L, with the dose per administration equaling to 1/10 of circulating blood volume. The mechanism of action consists of the oxidation of hydrophobic toxins by active oxygen. Sodium hypochlorite is a transporter of active oxygen which participates in phagocytosis and improves the detoxication function of the liver. Repeated analysis for antibiotic resistance was performed on the 3rd day of electrochemical detoxication.
Analysis of the biologic materials and interpretation of results of the microbiological investigations were performed according to the typical recommendations3, 7. We used Bergey’s classification of bacteria. After the primary isolation of the causative agent, we used K-disc-diffusion to identify antibiotic susceptibility (Kirby-Bauer)1.
Results
After the analysis of the obtained data, we found that the amount of Klebsiella pneumoniae strains isolated from group II patients decreased by 39 % (р ≤ 0.05), when compared to the group I patients (Figure 1).
After the assessment of Ps. Aeruginosa, we found that the prevalence of these microorganisms in group II patients decreased by 17 % (р ≤ 0.05), when compared to group I patients (Figure 2).
The level of Acinetobacter spp. increased by 25 % (р ≤ 0.05) in group II, which aligned with the tendencies and trends internationally (Figure 3).
After assessment of the antibiotic susceptibility of the studied strains, we found an increase in Acinetobacter spp. susceptibility to imipenem- from 16.7 % in group I patients to 100 % in group II patients (р ≤ 0.05).
Analysis of the susceptibility of Ps. aeruginosa showed that the susceptibility to meropenem remained at the same level (16.7 %) (р ≤ 0.05) (Table 1).
| Group I | Group II | ||||||
| Antibiotic | % R | % I | % S | Antibiotic | % R | % I | % S |
| Amikacin | 37.5 | 0 | 62.5 | Amikacin | 33.3 | 0 | 66.7 |
| Cefepime | 0 | 0 | 100 | Cefepime | 16.7 | 0 | 83.3 |
| Cefoperazone | 100 | 0 | 0 | Cefoperazone | 100 | 0 | 0 |
| Ceftazidime | 85.7 | 0 | 14.3 | Ceftazidime | 75 | 0 | 25 |
| Gatifloxacin | 100 | 0 | 0 | Ciprofloxacin | 100 | 0 | 0 |
| Gentamicin | 50 | 0 | 50 | Clindamycin | 100 | 0 | 0 |
| Imipenem | 50 | 10 | 40 | Gatifloxacin | 100 | 0 | 0 |
| Meropenem | 83.3 | 0 | 16.7 | Imipenem | 0 | 0 | 100 |
| Piperacillin | 0 | 66.7 | 33.3 | Levofloxacin | 100 | 0 | 0 |
| Tetraciclin | 0 | 0 | 0 | Linezolid | 100 | 0 | 0 |
| Tabromicin | 42.9 | 14.3 | 42.9 | Meropenem | 83.3 | 0 | 16.7 |
| - | - | - | - | Vancomicyn | 100 | 0 | 0 |
| - | - | - | - | Erapenem | 100 | 0 | 0 |
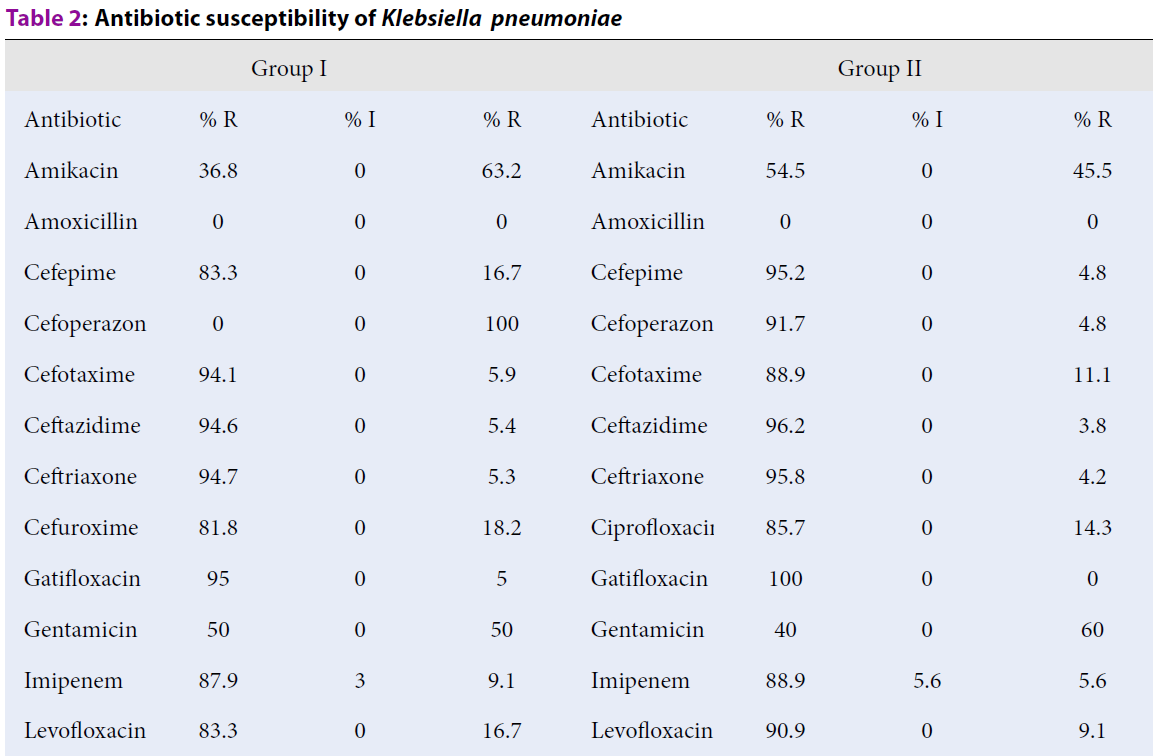
Susceptibility to amikacin and ceftazidime increased by 6.72 and 42.8 %, respectively (р ≤ 0.05). Taking into account the presence of polyresistant clinical isolates of Klebsiella pneumoniae in group I and II patients, a decrease of their amount by 39 % has a crucial prognostic meaning (Table 2).
| Group I | Group II | ||||||
| Antibiotic | % R | % I | % R | Antibiotic | % R | % I | % R |
| Amikacin | 36.8 | 0 | 63.2 | Amikacin | 54.5 | 0 | 45.5 |
| Amoxicillin | 0 | 0 | 0 | Amoxicillin | 0 | 0 | 0 |
| Cefepime | 83.3 | 0 | 16.7 | Cefepime | 95.2 | 0 | 4.8 |
| Cefoperazone | 0 | 0 | 100 | Cefoperazone | 91.7 | 0 | 4.8 |
| Cefotaxime | 94.1 | 0 | 5.9 | Cefotaxime | 88.9 | 0 | 11.1 |
| Ceftazidime | 94.6 | 0 | 5.4 | Ceftazidime | 96.2 | 0 | 3.8 |
| Ceftriaxone | 94.7 | 0 | 5.3 | Ceftriaxone | 95.8 | 0 | 4.2 |
| Cefuroxime | 81.8 | 0 | 18.2 | Ciprofloxacin | 85.7 | 0 | 14.3 |
| Gatifloxacin | 95 | 0 | 5 | Gatifloxacin | 100 | 0 | 0 |
| Gentamicin | 50 | 0 | 50 | Gentamicin | 40 | 0 | 60 |
| Imipenem | 87.9 | 3 | 9.1 | Imipenem | 88.9 | 5.6 | 5.6 |
| Levofloxacin | 83.3 | 0 | 16.7 | Levofloxacin | 90.9 | 0 | 9.1 |
| Meropenem | 95.8 | 0 | 4.2 | Meropenem | 83.3 | 0 | 16.7 |
| Ofloxacin | 94.7 | 0 | 5.3 | Ofloxacin | 50 | 0 | 50 |
| Ertapenem | 66.7 | 0 | 33.3 | Ertapenem | 84.6 | 0 | 15.4 |
| Tigecycline | 0 | 0 | 0 | Piperacillin | 92.3 | 0 | 7.7 |
| Piperacillin | 83.3 | 11.1 | 5.6 | Tetracycline | 0 | 0 | 0 |
| Tetracycline | 0 | 0 | 0 | Tobramycin | 65 | 0 | 35 |
| Tobramycin | 17.4 | 8.7 | 73.9 | Ticarcillin | 0 | 0 | 0 |
| Aztreonam | 100 | 0 | 0 | ||||
| Ticarcillin | 0 | 0 | 0 | ||||
Increase of antibiotic susceptibility of clinical isolates of Klebsiella pneumoniae to gentamycin and ofloxacin was 16.7 % and 89.4 %, respectively (р ≤ 0.05) (Table 2). A tendency towards an increase of antibiotic susceptibility of clinical strains of Acinetobacter spp. to meropenem was found. The increased susceptibility was from 0 to 30 % (р ≤ 0.05) (Table 3).
| Group I | Group II | ||||||
| Antibiotic | % R | % I | % S | Antibiotic | % R | % I | % S |
| Amikacin | 80 | 0 | 20 | Amikacin | 85.7 | 14.3 | 0 |
| Cefoperazone | 100 | 0 | 0 | Amoxicillin | 100 | 0 | 0 |
| Ceftazidime | 92.3 | 0 | 7.7 | Cefepime | 90 | 0 | 10 |
| Gatifloxacin | 80 | 0 | 20 | Cefoperazone | 84.6 | 0 | 15.4 |
| Gentamicin | 100 | 0 | 0 | Ceftazidime | 93.3 | 0 | 6.7 |
| Imipenem | 83.3 | 0 | 16.7 | Ciprofloxacin | 100 | 0 | 0 |
| Levofloxacin | 100 | 0 | 0 | Gatifloxacin | 75 | 8.3 | 16.7 |
| Meropenem | 80 | 20 | 0 | Gentamicin | 50 | 0 | 50 |
| Ertapenem | 0 | 0 | 0 | Imipenem | 0 | 0 | 100 |
| Tigecycline | 0 | 0 | 0 | Kanamycin | 100 | 0 | 0 |
| Piperacillin | 25 | 75 | 0 | Levofloxacin | 92.5 | 0 | 7.1 |
| Tetracycline | 0 | 0 | 0 | Meropenem | 70 | 0 | 30 |
| Tobramycin | 50 | 0 | 0 | Ertapenem | 0 | 0 | 0 |
| Ticarcillin | 0 | 0 | 100 | Piperacillin | 80 | 20 | 0 |
| Tetracycline | 0 | 0 | 0 | ||||
| Tobramycin | 75 | 0 | 25 | ||||
| Ticarcillin | 100 | 0 | 0 | ||||
Discussion
The most significant changes of antibiotic susceptibility were determined for imipenem. It increased by 60 % (р ≤ 0.05), which was 100 % susceptibility of clinical isolates of Ps. aeruginosa to imipenem. Taking into account the remaining polyresistant clinical isolates of Klebsiella pneumoniae in group I and II patients, a decrease of their amounts by 39 % has an outstanding prognostic value.
Acinetobacter spp. was not susceptible to cefoperazone/sulbactam in group I patients, while 15.4 % of strains were susceptible to this antibiotic in the group II patients. Data on some of the antibiotics correlate with other studies conducted in our country8.
Conclusions
A complex of proposed measures included the division of patients in blocks according to the risk of infectious complications, control of antibiotics administration, adherence to sanitary norms by ICU staff, use of sodium hypochlorite (which leads to the decrease of pathogenic isolates), and level of antibiotic resistance to specific groups of antibacterial drugs. The complex measures caused a decrease in the amount of pathogenic isolates and in antibiotic resistance levels to certain antibiotics without high expenses.
Assessment of the microbiological scope of the departments with antibiotic susceptibility analysis of isolated clinical isolates should be an obligatory part of the administration of the antibiotic.
Abbreviations
ICU: Intensive care unit
Acknowledgments
Not applicable.
Author’s contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
Not applicable.
Availability of data and materials
Data and materials used and/or analysed during the current study are available from the corresponding author on reasionable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Jarrett-Wilkins
C.N.,
R.A. Musgrave,
R.L.N. Hailes,
Harniman
R.L.,
Faul
C.F.J.,
Manners
I.,
Linear and Branched Fiber-like Micelles from the Crystallization-Driven Self-Assembly of Heterobimetallic Block Copolymer Polyelectrolyte/Surfactant Complexes. J Macromolecules.
2019;
52
(19)
:
7289-7300
.
View Article Google Scholar -
Georges
B.,
Conil
J.M.,
A. Dubouix,
M. Archambaud,
E. Bonnet,
S. Saivin,
Lauwers-Cancès
V. ,
Risk of Emergence of Pseudomonas Aeruginosa Resistance to Beta-lactam Antibiotics in Intensive Care Units. J Crit Care Med.
2006;
34
:
1636-1641
.
View Article PubMed Google Scholar -
J.C. Marshall,
R.V. Maier,
M. Jimenez,
Dellinger
E.P.,
Source Control in the Management of Severe Sepsis and Septic Shock: An Evidence-Based Review. J Crit Care Med.
2004;
32
:
513-526
.
View Article PubMed Google Scholar -
Amato-Gauci
A.,
Ammon
A.,
The First European Communicable Disease. Epidemiological Report. European Centre for Disease Prevention and Control. Stockholm.
2017
.
-
Mazuski
J.E.,
Sawyer
R.G.,
Nathens
A.B.,
DiPiro
J.T.,
Schein
M.,
Kudsk
K.A.,
C. Yowler,
Therapeutic Agents Committee of the Surgical Infections Society: The Surgical Infection Society Guidelines on Antimicrobial Therapy for Intra-abdominal Infections: an Executive Summary. J Surg Infect (Larchmt).
2002;
3
:
161-173
.
View Article PubMed Google Scholar -
O.A. Nazarchuk,
D.V. Dmytriiev,
K.D. Dmytriiev,
H.H. Nazarchuk,
D.V. Zaletskiy,
Characteristics of Infectious Complications in Critically Ill Patients. J Wiad Lek.
2018;
71
(9)
:
1784-1792.
.
-
Кollef
M.H.,
The Intensive Care Medicine Research Agenda on Multidrug-Resistant Bacteria, Antibiotics, and Stewardship. J Intensive Care Med.
2017
.
View Article PubMed Google Scholar -
Nahaichuk
V.I.,
Nazarchuk
O.A.,
Osadchuk
N.I.,
Dmytriiev
D.V.,
Nazarchuk
H.H.,
The Analytical Prognosis of the Susceptibility to Aminoglycosides and Doxycycline in Acinetobacter Baumanuu Usolated from Burns of Intensive Care Unit Patients. J Wiad Lek.
2018;
71
(3 pt 2)
:
705-709
.
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 7 (2020)
Page No.: 3860-3864
Published on: 2020-07-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4720 times
- Download PDF downloaded - 1694 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress