Abstract
Introduction: Alpha-2-macroglobulin (α2M) is large glycoprotein, found in the plasma of vertebrates and invertebrates, which plays a major role in regulation and transport. In this study, we report the interaction of 5-fluorouracil (5-FU) with α2M and the protective effect of quercetin against 5-FU induced modification of α2M.
Methods: Various biochemical and biophysical methods and techniques were employed to determine the binding interaction between α2M-5-FU and the preventive effect of quercetin on 5-FU induced modification of α2M. Fluorescence microscopy was performed to analyze the formation of free radicals by 5-FU and to examine the scavenging effect of quercetin.
Results: Our results of antiproteinase activity assay show that the 5-FU-α2M interaction causes functional inactivation of sheep α2M, while the 5-FU-α2M complex after exposure to quercetin regains its native conformation. Intrinsic fluorescence results suggest an increase of 5-FU quenching in the fluorescence intensity, and when the mixture was incubated with quercetin the α2M-FU-quercetin mixture showed the same intensity as that of native protein. The absorption spectra of 5-FU-α2M complex suggest the formation of a complex leading to increase in absorbance. CD and FTIR spectroscopy show 5-FU causes secondary structural alteration of α2M and quercetin provides a protective role against structural modification.
Conclusion: Our study suggests that 5-FU produces reactive oxygen species (ROS) in the presence of light and compromises the integrity of α2M. Quercetin quenches the ROS formed by 5-FU and helps the protein to maintain its native conformation. We have demonstrated the protective effect of quercetin for the first time against the toxicity induced by 5-FU on α2M.
Introduction
5-fluorouracil (5-FU), a fluorinated pyrimidine analogue, is an anticancer drug and is an analogue of uracil containing fluorine at C-5 position instead of hydrogen atom1. 5-FU can be incorporated into DNA and RNA due its structure and the fact that it interferes with nucleoside metabolism, which may lead to cell death and cytotoxicity2. 5-FU is found to be a beneficial drug used for the treatment of tumours of the head, breast and neck3. It is also an effective drug to treat cancers of the gastrointestinal tract, stomach, cervix and colon4, 5, 6, 7. However, it is also associated with toxicity, the most common being acute coronary syndrome8, 9, and the administration of 5-FU to humans can induce cardiotoxic effects such as cardiomyopathy and vasospastic angina10, 11, 12, 13, 14. The interaction of anticancer drugs with proteins is very effective in pharmacology and is considered to be important for the administration of drugs in the human body.
Sheep alpha-2-macroglobulin (α2M) is a large tetrameric glycoprotein of 630 kDa15, containing identical subunits joined by non-covalent disulphide bonds16, 17. The α2M has been isolated from various sources such as haemolymph of invertebrates, plasma of vertebrates and the egg white of birds and reptiles18, 19, 20, 21, 22. It is a major antiproteinase found in the blood and tissues and binds to various cytokines and growth factors23 and is also responsible for the binding and transport of human growth hormone24. It is a classic proteinase inhibitor and can inhibit all proteinases irrespective of their specificity and catalytic mechanism25, 26; however, it does not inactivate them, which makes it unique. After inhibition, it obstructs the entry of substrates (large molecular weight) to the active site27.
Flavonoids are the compounds of plant origin and possess antioxidant and anti-inflammatory properties. Quercetin provides a protective effect against free radicals and also plays a vital role in the reduction of oxidative stress28, 29, 30, 31, 32. The aim of this study is to investigate the antioxidant effect of quercetin and its protective effect on 5-FU-induced modification of α2M. Several studies show that 5-FU produces free radicals in the presence of light, which disrupts the integrity of proteins32, 33, 34, 35, 36, 37. When 5-FU interacts with α2M in the presence of light it induces a change in α2M both structurally and functionally. In the present study, we provide evidence for the binding of 5-FU with α2M. Moreover, we also provide information regarding the protective effect of quercetin against the toxicity induced by 5-FU on α2M.
Methods
Materials
Trypsin, soya bean trypsin inhibitor (STI), N-Benzoyl-DL-arginine-p-nitroanilide (BAPNA) and Sephacryl 300HR, quercetin, 5-FU and quercetin were obtained from Sigma-Aldrich, India. All reagents used were analytical grade and commercially available.
Methods
α2M antiproteinase activity
To analyse the change in the functionality of α2M, antiproteinase assay was performed. The activity profile of protein was measured with increasing concentrations of 5-FU in the presence of light. To determine the protective effect of quercetin, it was preincubated with protein and then treated with 5-FU38. The reaction mixture consisted of 1 ml sample in 50 mM sodium phosphate buffer (pH 7.4) at room temperature (22 ± 1 0C). After incubation for 1 hr, 0.161 nmol trypsin was added into the mixture and incubated for 15 min at 37ºC. After 15 min incubation, 0.45 nmol of soybean trypsin inhibitor (STI) was added into the reaction mixture and the incubation was continued for additional 15 min at 37ºC. Finally, 2.28 μmol of N-Benzoyl-DL-arginine-p-nitroanilide (BAPNA) was added and absorbance was observed at 410 nm.
Intrinsic fluorescence measurements
The fluorescence spectra for native α2M and 5-FU and quercetin were recorded on a Shimadzu RF-5301 spectrofluorometer (Tokyo, Japan) in a 10 mm path length quartz cell. The excitation wavelength was set at 280 nm and the emission spectra were recorded in the range of 300-400. The concentration of protein in the sample was 10 µM with varying concentration of 5-FU and the concentration of quercetin was 50 µM in the aliquot.
Synchronous fluorescence analysis
Synchronous fluorescence was recorded on a Shimadzu RF5301 spectrofluorophotometer, Japan. The wavelength range (250 – 400 nm) of fluorescence spectra selected for synchronous scanning was recorded at Δλ of 15 nm and 60 nm for tyrosine and tryptophan, respectively.
UV absorption spectroscopy
UV spectrum of native α2M (10 µM) and α2M incubated with quercetin (50 µM) with increasing concentration of 5-FU were recorded between 250 – 350 nm on a Shimadzu UV visible Spectrophotometer UV-1700 using a cuvette of 1 cm path length39.
FTIR spectroscopy
FTIR spectroscopy was performed on an Interspec 2020 FTIR spectrometer. The samples were prepared by dissolving native α2M (20 µM) and α2M incubated with quercetin and 5-FU in 20 mM sodium phosphate buffer, pH 7.4. The spectra of native α2M incubated with varying concentration of 5-FU and quercetin (50 µM) were analyzed. Amide I (1600 – 1700 cm-1) is the region which is usually analyzed to confirm the change in the native structure of the protein. The scanning wave number was 1000 – 4000 cm-140.
CD measurements
CD measurements were recorded on a JASCO-J815 spectropolarimeter equipped with a Peltier-temperature controller. Wavelength scan for native α2M (20 µM) and quercetin with varying concentration of 5-FU were carried out. The temperature was maintained at 25°C and the speed was fixed at 100 nm/min with a response time of 1s. Each spectrum was the average of 3 scans and was recorded in the wavelength range of 200 – 250 nm using quartz cuvette of 0.1 cm path length.
Measurement of superoxide generation by 5-FU
The superoxide species (O2.-) generation was estimated by the nitro-blue-tetrazolium (NBT) assay41. A typical assay mixture was used containing 50 mM sodium phosphate buffer (pH 8.0), 300 µM NBT, 100 µM EDTA and 0.06% Triton-X-100 and α2M (20 µM) treated with varying concentration of 5-FU (10 – 50 µM). The absorbance was recorded at 560 nm at different time intervals, against a blank which was without 5-FU.
Fluorescence microscopy
Fluorescence microscopy was utilized to determine the formation of reactive oxygen species (ROS). Determination of ROS was performed using the dichlorofluorescein (DCF) method42. Protein was preincubated with quercetin for 1 hr, treated with 5-FU and incubated in the presence of white light. Then the sample was incubated with 10 μM dichloro-dihydro-fluorescein diacetate (DCFH-DA) for 1 hr at 37°C. The treated protein was placed on glass coverslips and visualized under a fluorescent microscope at 40 x magnification.
Statistical Analysis
Results were shown as the mean and the standard deviation (SD values, with n=3 as the number of independent experiments.
Results
Antiproteinase activity
The activity assay of native α2M with varying concentration of 5-FU was measured in order to check the functional status of the protein. Firstly, we measured the antiproteinase activity of protein incubated with the drug in the presence of white light. Our results show that the activity of protein decreases with increasing concentration of 5-FU (10 – 50 µM). According to Figure 1a, the residual activity was found to be 87% at 10 µM 5-FU. When the concentration of the drug (50 µM) was increased, the remaining activity was found to be 48%. According to previous studies, 5-FU is known to generate free radicals in the presence of white light43. Therefore, the anti-proteolytic potential of the protein was compromised, which may be due to the formation of free radicals by the drug leading to changes in the functionality of the protein. We measured the protective effect of quercetin against the toxicity induced by 5-FU, since it is a flavonoid which is known to scavenge free radicals44. When the protein was preincubated with quercetin and then treated with 5-FU in the presence of light the resultant activity decreased, but not as much compared to 5-FU alone. Our results suggest that the residual inhibitory activity at 50 µM 5-FU was 59% when the protein was preincubated with quercetin (Figure 1b). Therefore, quercetin provides a protective role against 5-FU since it may scavenge free radicals that are generated by the drug.
Intrinsic fluorescence measurements
Intrinsic fluorescence is mainly used to determine the conformational changes in the native form of the protein. The fluorescence spectra were recorded for the protein (20 µM) with increasing concentration of 5-FU (10 – 50 µM). The protein was excited at 280 nm and emission spectra were taken in the range 300 - 400 nm. The emission maximum (λmax/λem) for native α2M was found to be 328 nm, as shown in Figure 2a. It is quite clear from the figure that with increasing concentration of 5-FU, the fluorescence intensity decreases continuously with a blue shift of 3 nm. The decrease in the intensity is due to the quenching in fluorescence due to the interaction between the protein and the drug. The quenching occurs mainly due to three intrinsic fluorophores in the protein i.e., phenylalanine, tyrosine and tryptophan45. The change in the fluorescence intensity can be correlated to the change in the structure of α2M as the ligand binds to it46, which may also alter the function of the protein. Many in vitro studies suggest that quercetin can reduce the toxicity induced by anticancer drugs and provide a protective effect against free radical induced damage31. To evaluate the protective effect of quercetin against 5-FU-induced toxicity on α2M, intrinsic fluorescence was recorded. The protein was first preincubated with quercetin and then treated with the drug in the presence of white light, intrinsic fluorescence was then recorded. As the results suggest, with varying concentrations of 5-FU the fluorescence intensity decreases, but when the protein was preincubated with quercetin, less quenching occurs (Figure 2b). So, we can conclude that in the presence of quercetin, α2M regains its native structure which was damaged by the drug.
Synchronous fluorescence analysis
Synchronous fluorescence was performed to study the changes in the molecular environment in the vicinity of the chromophore. The spectral analysis provides information about the conformational changes around the tyrosine and tryptophan residues of the protein. The effect of fluorescence analysis of 5-FU on α2M is shown in Figure 3. With the addition of drug, there was a decrease in the fluorescence intensity with a shifting of emissions towards a longer wavelength from 341 to 344 nm for tryptophan residues and towards a shorter wavelength from 309 to 305 nm for tyrosine residues. This decrease in the fluorescence intensity with varying concentration of 5-FU suggests changes in the surrounding microenvironment around tyrosine and tryptophan residues with a decrease in hydrophobicity around both residues47.
UV absorption spectroscopy
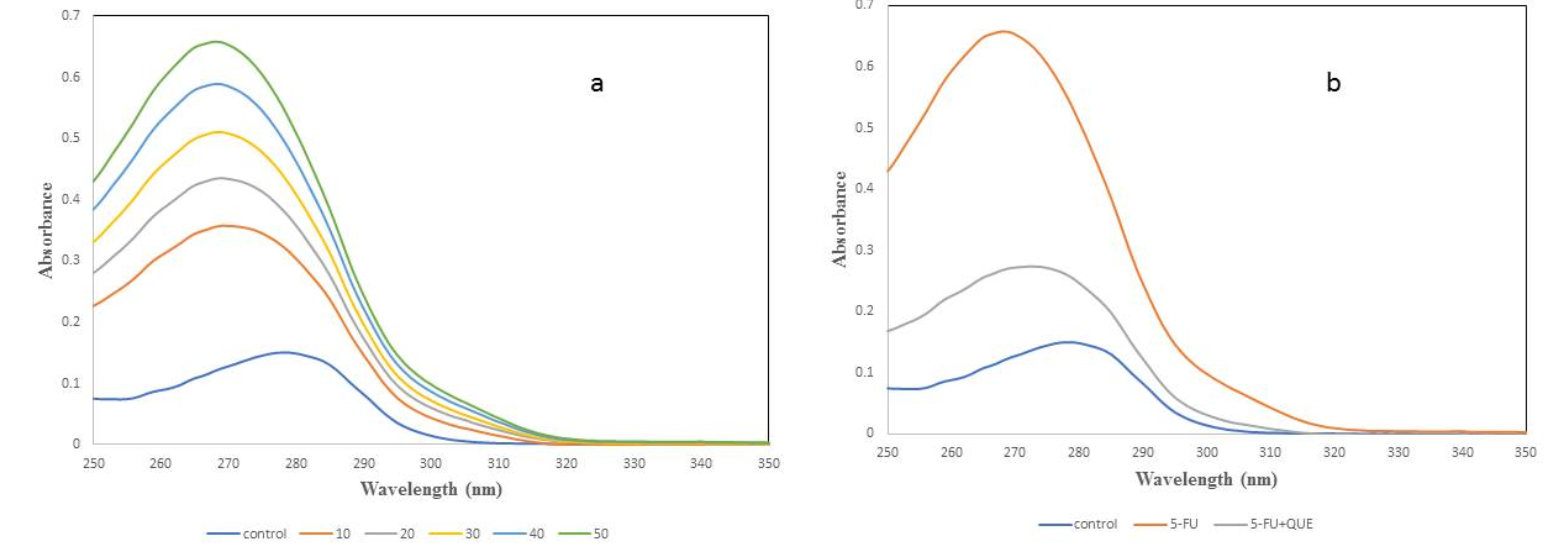
UV spectra was analyzed to determine the structural change in the native protein and to study the formation of a complex between protein and ligand48. It is a classical technique to determine the modification in the microenvironment of amino acid residues, particularly tryptophan, resulting in the change in the spectra or a shift in maximum absorbance/lambda max (λmax)45. To examine the change in the native structure of α2M with varying concentration of 5-FU, spectra was recorded between 250 – 350 nm (Figure 4a). As shown in Figure 4, the λmax for native α2M was found to be 278 nm, but with increasing concentration of 5-FU, the absorbance increases and a blue shift (10 nm) was observed. The blue shift indicates that the interaction of drug with α2M causes some structural changes in the native form of protein. It is well-known that 5-FU forms free radicals in the presence of white light that may cause damage or change in the protein. So, quercetin was used as a protective agent to scavenge the free radicals formed by the drug. α2M was preincubated with quercetin and then treated with 5-FU in the presence of light. When α2M was preincubated with quercetin, the effect of drug was less compared to drug alone and λmax was found to be 275 nm (Figure 4b). Our results suggest that quercetin scavenges free radicals formed by drug and prevents oxidative damage and/or restores the native structure of protein.
Measurement of superoxide generation by 5-FU
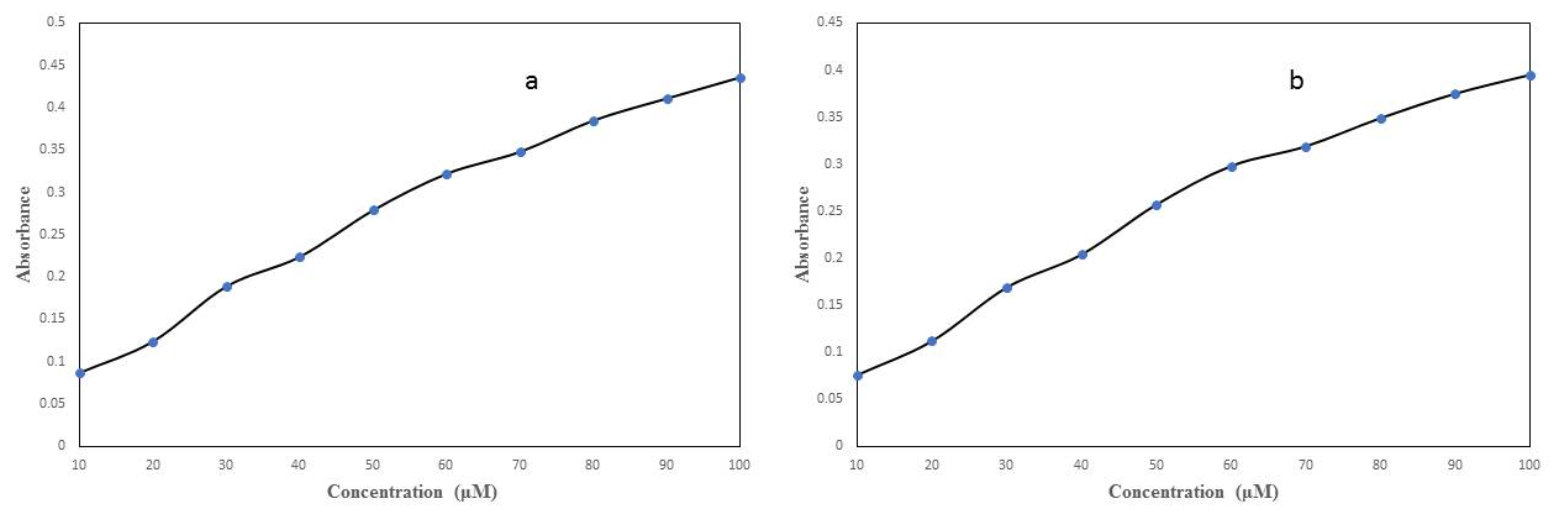
5-FU was subjected for photo-illumination in order to measure the free radical(s) generated by it. The free radical generated by drug was measured by the NBT reduction assay.Figure 5a depicts the generation of superoxide anions (O2.-) with increasing concentration of 5-FU (10 – 100 µM). α2M was preincubated with quercetin (100 µM) and then exposed to 5-FU for 1 hr in light. According to Figure 5b, quercetin decrease the formation of superoxide anion as compared to 5-FU alone. So, the results suggest that quercetin has antioxidant activity against the formation of free radicals by 5-FU.
FTIR spectroscopy
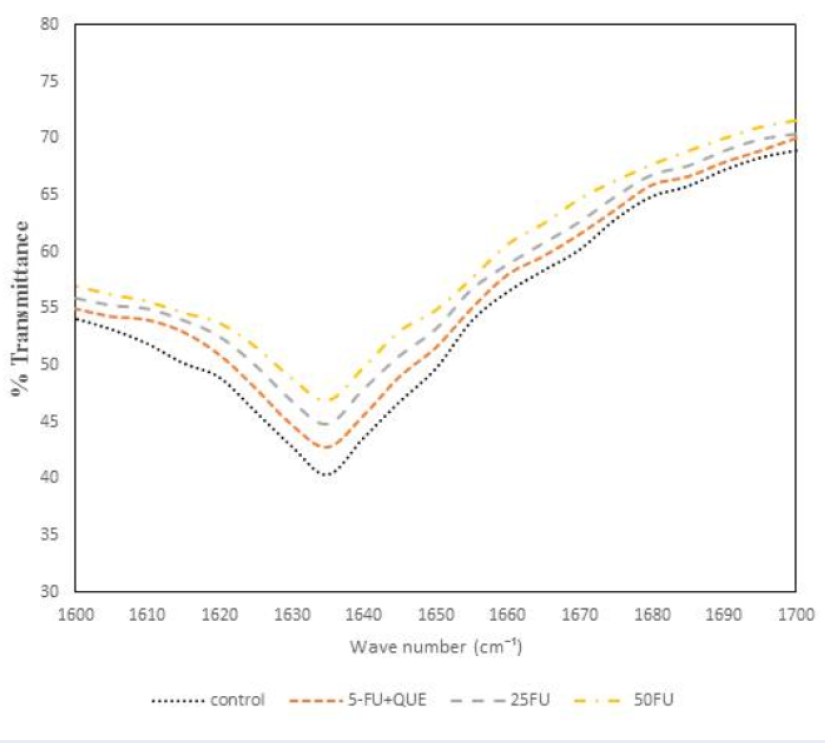
The FTIR spectroscopy was utilized to determine the change in the secondary structure of protein. Amide I band was analyzed as it is the most important protein band for the analysis of possible structural changes. The amide I band displays in the wave number range 1600 – 1700 cm-1 governed by the stretching of the C=O (70 — 85%) and minor contribution of C-N groups (10 — 20%).Figure 6 shows the FTIR spectra of native α2M along with 5-FU and quercetin. The amide I band for native α2M was centered at around 1635 cm-1 which is characteristic of β-sheet structure38. However, upon incubation with 5-FU, the amide I band was at 1635 cm-1, but the intensity decreases with varying concentration which implies the loss of protein native conformation. The native conformation of α2M is lost, which affects the functional activity of the antiproteinase inhibitor suggesting that 5-FU causes structural changes in the native conformation of protein, which was confirmed by the antiproteinase activity results. In order to determine the preventive effect of quercetin, the protein was incubated with 5-FU. Figure 6 shows that the band for α2M preincubated with quercetin was found to be close to that of native protein. Hence, quercetin scavenges the free radicals from the drug and provides a protective role against the oxidative modification of α2M.
CD measurements
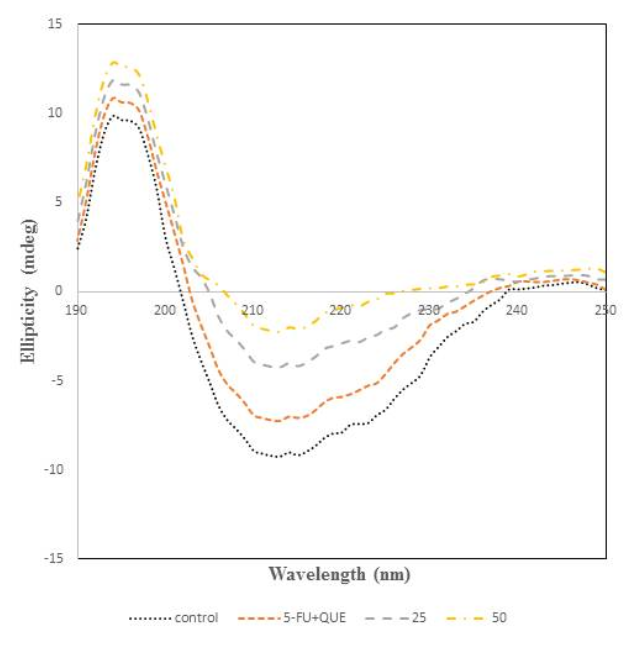
CD spectroscopy was performed to analyse the structural transitions in the structure of protein since it gives information regarding the secondary structural changes49, 50, 51. The CD spectra of α2M incubated with 5-FU and quercetin are shown in Figure 7. The peak of native protein was found at 215 nm which is a characteristic feature of β-sheet structure38, 39. However, varying the concentration of 5-FU leads to changes in the far UV-CD spectra resulting in the increase, which suggests the loss of β-sheet content in native α2M. So, the formation of free radicals by the drug causes structural distortion in the native form of protein. Thus, it can be said that the loss in β-sheet content of the protein alters the biological activity of the protein. In order to identify the protective effect of quercetin, it was incubated with α2M and 5-FU and it was found that α2M preincubated with quercetin shows spectra very similar to that of native protein.
Fluorescence microscopy
The effect of drug on the native protein and effect of quercetin on 5-FU treated protein was examined. As depicted inFigure 8a, the native protein shows no fluorescence which indicates no free radicals are present in the sample. 5-FU is known to form free radicals in the presence of white light which can damage the native conformation of protein. When α2M was incubated with 5-FU (50 µM) it showed bright fluorescence representing the production of free radicals (Figure 8b). When α2M was preincubated with quercetin and then treated with 5-FU, a weaker fluorescence was observed, which shows a reduction in the formation of free radicals (Figure 8c).
Discussion
The flavonoids are the polyphenolic compounds of plant origin found in vegetables, fruits, red wine, tea, etc. and possess antioxidant and anti-inflammatory properties28, 29, 30, 31. Quercetin provides a protective effect against free radicals induced oxidative stress and possesses many beneficial effects against liver disease that may be caused by the endotoxins32, 33, 34, 35, 36, 37. In the present study, the interaction of 5-FU with α2M was examined for the first time. Quercetin was incubated with 5-FU treated α2M and antiproteinase activity assay was performed to measure the functional activity of the protein. 5-FU causes structural and functional changes in the native conformation of α2M and quercetin was used to prevent the toxic effects of the drug. The results obtained from the intrinsic fluorescence and absorption spectroscopy show that 5-FU causes conformation changes in the native form of protein due to the formation of free radicals and quercetin provided a protective role. CD and FTIR spectroscopy were used to analyze the secondary structural changes in the protein treated with the drug and it regained its native conformation in the presence of quercetin. Fluorescence microscopy was analyzed to study the formation of free radicals by 5-FU, the results show that 5-FU forms free radicals and quercetin provides a scavenging role. On the other hand, when the α2M-5FU mixture was treated with quercetin, the protein regains its native conformation. Our study provides an insight into the 5-FU-α2M interaction and the scavenging role of quercetin.
Conclusions
In the present study, we reported the interaction of 5-FU with α2M and the protective effect of quercetin against 5-FU induced modification of α2M. Various biochemical and biophysical methods were employed to determine the binding between α2M-5-FU and the protective effect of quercetin on 5-FU induced modification of α2M. Photoilluminated 5-FU produces ROS which compromises the structural and functional integrity of α2M. Quercetin quenches the ROS produced by 5-FU and helps the protein to maintain its native conformation. Therefore, we demonstrated the protective effect of quercetin for the first time against the toxicity induced by 5-FU on α2M.
Abbreviations
5-FU: 5-fluorouracil, α2M: alpha-2-macroglobulin, CD: circular dichroism, FTIR: fluorescence transform infra-red spectroscopy, ROS: reactive oxygen species
Acknowledgments
The authors are grateful to the Department of Biochemistry, Aligarh Muslim University, Aligarh for the facilities provided. The University Grants Commission (UGC), New Delhi, is also acknowledged for funding. We wish to thank the reviewers for their opinion, suggestions and recommendation.
Author’s contributions
SSA: collected data and performed analysis; acquisition, analysis and interpretation of data; drafted the manuscript; HA: wrote the paper; drafted the manuscript; critically revised the manuscript; SA: performed the analysis; FHK: conceived, conceptualized and designed the study; gave final approval.
Funding
The University Grants Commission (UGC), New Delhi.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Peters
G.J.,
Laurensse
E.,
Leyva
A.,
Lankelma
J.,
Pinedo
H.M.,
Sensitivity of human, murine, and rat cells to 5-fluorouracil and 5'-deoxy-5-fluorouridine in relation to drug-metabolizing enzymes. Cancer Research.
1986;
46
(1)
:
20-8
.
PubMed Google Scholar -
Zhang
N.,
Yin
Y.,
Xu
S.J.,
Chen
W.S.,
5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules (Basel, Switzerland).
2008;
13
(8)
:
1551-69
.
View Article PubMed Google Scholar -
Morris
S.M.,
The genetic toxicology of 5-fluoropyrimidines and 5-chlorouracil. Mutation Research.
1993;
297
(1)
:
39-51
.
View Article PubMed Google Scholar -
Wang
X.,
Lin
J.,
Zhang
X.,
Liu
Q.,
Xu
Q.,
Tan
R.X.,
5-Fluorouracil-cisplatin adducts with potential antitumor activity. Journal of Inorganic Biochemistry.
2003;
94
(1-2)
:
186-92
.
View Article PubMed Google Scholar -
Lamberti
M.,
Porto
S.,
Marra
M.,
Zappavigna
S.,
Grimaldi
A.,
Feola
D.,
5-Fluorouracil induces apoptosis in rat cardiocytes through intracellular oxidative stress. Journal of Experimental {&}amp; Clinical Cancer Research.
2012;
31
(1)
:
60
.
View Article PubMed Google Scholar -
Pendekal
M.S.,
Tegginamat
P.K.,
Development and characterization of chitosan-polycarbophil interpolyelectrolyte complex-based 5-fluorouracil formulations for buccal, vaginal and rectal application. Daru : Journal of Faculty of Pharmacy, Tehran University of Medical Sciences.
2012;
20
(1)
:
67
.
View Article PubMed Google Scholar -
Steger
F.,
Hautmann
M.G.,
Kölbl
O.,
5-FU-induced cardiac toxicity\textemdashan underestimated problem in radiooncology?. Radiation Oncology (London, England).
2012;
7
(1)
:
212
.
View Article PubMed Google Scholar -
Kosmas
C.,
Kallistratos
M.S.,
Kopterides
P.,
Syrios
J.,
Skopelitis
H.,
Mylonakis
N.,
Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. Journal of Cancer Research and Clinical Oncology.
2008;
134
(1)
:
75-82
.
View Article PubMed Google Scholar -
Labianca
R.,
Beretta
G.,
Clerici
M.,
Fraschini
P.,
Luporini
G.,
Cardiac toxicity of 5-fluorouracil: a study on 1083 patients. Tumori.
1982;
68
(6)
:
505-10
.
View Article PubMed Google Scholar -
Jensen
S.A.,
S∅rensen
J.B.,
Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemotherapy and Pharmacology.
2006;
58
(4)
:
487-93
.
View Article PubMed Google Scholar -
Mosseri
M.,
Fingert
H.J.,
Varticovski
L.,
Chokshi
S.,
Isner
J.M.,
In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Research.
1993;
53
(13)
:
3028-33
.
PubMed Google Scholar -
Stewart
T.,
Pavlakis
N.,
Ward
M.,
Cardiotoxicity with 5-fluorouracil and capecitabine: more than just vasospastic angina. Internal Medicine Journal.
2010;
40
(4)
:
303-7
.
View Article PubMed Google Scholar -
Saif
M.W.,
Shah
M.M.,
Shah
A.R.,
Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opinion on Drug Safety.
2009;
8
(2)
:
191-202
.
View Article PubMed Google Scholar -
Sorrentino
M.F.,
Truesdell
A.G.,
5-Fluorouracil-induced coronary thrombosis: A case report and review of the literature. Journal of Cardiology Cases.
2012;
6
(1)
:
e20-2
.
View Article PubMed Google Scholar -
Rehman
A.A.,
Ahsan
H.,
Khan
F.H.,
Identification of a new alpha-2-macroglobulin: multi-spectroscopic and isothermal titration calorimetry study. International Journal of Biological Macromolecules.
2016;
83
:
366-75
.
View Article PubMed Google Scholar -
Barrett
A.J.,
Brown
M.A.,
Sayers
C.A.,
The electrophoretically `slow' and `fast' forms of the alpha 2-macroglobulin molecule. The Biochemical Journal.
1979;
181
(2)
:
401-18
.
View Article PubMed Google Scholar -
Sottrup-Jensen
L.,
Alpha-macroglobulins: structure, shape, and mechanism of proteinase complex formation. The Journal of Biological Chemistry.
1989;
264
(20)
:
11539-42
.
View Article PubMed Google Scholar -
Buresova
V.,
Hajdusek
O.,
Franta
Z.,
Sojka
D.,
Kopacek
P.,
IrAM-An α2-macroglobulin from the hard tick Ixodes ricinus: characterization and function in phagocytosis of a potential pathogen Chryseobacterium indologenes. Developmental and Comparative Immunology.
2009;
33
(4)
:
489-98
.
View Article PubMed Google Scholar -
Raymond
W.W.,
Su
S.,
Makarova
A.,
Wilson
T.M.,
Carter
M.C.,
Metcalfe
D.D.,
α 2-macroglobulin capture allows detection of mast cell chymase in serum and creates a reservoir of angiotensin II-generating activity. Journal of Immunology (Baltimore, Md.: 1950).
2009;
182
(9)
:
5770-7
.
View Article PubMed Google Scholar -
Ma
H.,
Wang
B.,
Zhang
J.,
Li
F.,
Xiang
J.,
Multiple forms of alpha-2 macroglobulin in shrimp Fenneropenaeus chinesis and their transcriptional response to WSSV or Vibrio pathogen infection. Developmental and Comparative Immunology.
2010;
34
(6)
:
677-84
.
View Article PubMed Google Scholar -
Lim
W.,
Jeong
W.,
Kim
J.H.,
Lee
J.Y.,
Kim
J.,
Bazer
F.W.,
Differential expression of alpha 2 macroglobulin in response to dietylstilbestrol and in ovarian carcinomas in chickens. Reproductive Biology and Endocrinology.
2011;
9
(1)
:
137
.
View Article PubMed Google Scholar -
Neves
D.,
Estrozi
L.F.,
Job
V.,
Gabel
F.,
Schoehn
G.,
Dessen
A.,
Conformational states of a bacterial α2-macroglobulin resemble those of human complement C3. PLoS One.
2012;
7
(4)
:
e35384
.
View Article PubMed Google Scholar -
Borth
W.,
Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. The FASEB Journal.
1992;
6
(15)
:
3345-53
.
View Article PubMed Google Scholar -
Kratzsch
J.,
Selisko
T.,
Birkenmeier
G.,
Identification of transformed alpha 2-macroglobulin as a growth hormone-binding protein in human blood. The Journal of Clinical Endocrinology and Metabolism.
1995;
80
(2)
:
585-90
.
PubMed Google Scholar -
Khan
S.A.,
Khan
F.H.,
Oxidized caprine alpha-2-macroglobulin: damaged but not completely dysfunctional. Biochimica et Biophysica Acta.
2004;
1674
(2)
:
139-48
.
PubMed Google Scholar -
Lin
Z.,
Lo
A.,
Simeone
D.M.,
Ruffin
M.T.,
Lubman
D.M.,
An N-glycosylation analysis of human alpha-2-macroglobulin using an integrated approach. Journal of Proteomics {&}amp; Bioinformatics.
2012;
5
:
127-34
.
View Article PubMed Google Scholar -
Rehman
A.A.,
Ahsan
H.,
Khan
F.H.,
α-2-Macroglobulin: a physiological guardian. Journal of Cellular Physiology.
2013;
228
(8)
:
1665-75
.
View Article PubMed Google Scholar -
Kao
T.K.,
Ou
Y.C.,
Lin
S.Y.,
Pan
H.C.,
Song
P.J.,
Raung
S.L.,
Luteolin inhibits cytokine expression in endotoxin/cytokine-stimulated microglia. The Journal of Nutritional Biochemistry.
2011;
22
(7)
:
612-24
.
View Article PubMed Google Scholar -
Kao
T.K.,
Ou
Y.C.,
Raung
S.L.,
Lai
C.Y.,
Liao
S.L.,
Chen
C.J.,
Inhibition of nitric oxide production by quercetin in endotoxin/cytokine-stimulated microglia. Life Sciences.
2010;
86
(9-10)
:
315-21
.
View Article PubMed Google Scholar -
Fang
Y.Z.,
Yang
S.,
Wu
G.,
Free radicals, antioxidants, and nutrition. Nutrition (Burbank, Los Angeles County, Calif.).
2002;
18
(10)
:
872-9
.
View Article PubMed Google Scholar -
Çelik
H.,
Arinç
E.,
Evaluation of the Protective Effects of Quercetin, Rutin, Resveratrol, Naringenin and Trolox Against Idarubicin-Induced DNA Damage. Journal of Pharmacy {&}amp; Pharmaceutical Sciences.
2010;
13
(2)
:
231-41
.
View Article PubMed Google Scholar -
Lin
S.Y.,
Wang
Y.Y.,
Chen
W.Y.,
Chuang
Y.H.,
Pan
P.H.,
Chen
C.J.,
Beneficial effect of quercetin on cholestatic liver injury. The Journal of Nutritional Biochemistry.
2014;
25
(11)
:
1183-95
.
View Article PubMed Google Scholar -
Bharrhan
S.,
Chopra
K.,
Arora
S.K.,
Toor
J.S.,
Rishi
P.,
Down-regulation of NF-κB signalling by polyphenolic compounds prevents endotoxin-induced liver injury in a rat model. Innate Immunity.
2012;
18
(1)
:
70-9
.
View Article PubMed Google Scholar -
Choi
K.C.,
Chung
W.T.,
Kwon
J.K.,
Yu
J.Y.,
Jang
Y.S.,
Park
S.M.,
Inhibitory effects of quercetin on aflatoxin B1-induced hepatic damage in mice. Food and Chemical Toxicology.
2010;
48
(10)
:
2747-53
.
View Article PubMed Google Scholar -
de David
C.,
Rodrigues
G.,
Bona
S.,
Meurer
L.,
González-Gallego
J.,
Tuñón
M.J.,
Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicologic Pathology.
2011;
39
(6)
:
949-57
.
View Article PubMed Google Scholar -
Domitrović
R.,
Jakovac
H.,
Vasiljev Marchesi
V.,
Vladimir-Kne\vzević
S.,
Cvijanović
O.,
Tadić
Z.,
Differential hepatoprotective mechanisms of rutin and quercetin in CCl(4)-intoxicated BALB/cN mice. Acta Pharmacologica Sinica.
2012;
33
(10)
:
1260-70
.
View Article PubMed Google Scholar -
Larson
A.J.,
Symons
J.D.,
Jalili
T.,
American Society for Nutrition
Therapeutic potential of quercetin to decrease blood pressure: review of efficacy and mechanisms. Advances in Nutrition.
2012;
3
(1)
:
39-46
.
View Article PubMed Google Scholar -
Ali
S.S.,
Zia
M.K.,
Siddiqui
T.,
Ahsan
H.,
Khan
F.H.,
Biophysical analysis of interaction between curcumin and alpha-2-macroglobulin. International Journal of Biological Macromolecules.
2019;
128
:
385-90
.
View Article PubMed Google Scholar -
Ali
S.S.,
Zia
M.K.,
Siddiqui
T.,
Khan
F.H.,
Binding interaction of sheep alpha-2-macroglobulin and tannic acid: A spectroscopic and thermodynamic study. Spectrochimica Acta. Part A: Molecular and Biomolecular Spectroscopy.
2018;
204
:
748-53
.
View Article PubMed Google Scholar -
Marcon
G.,
Plakoutsi
G.,
Canale
C.,
Relini
A.,
Taddei
N.,
Dobson
C.M.,
Amyloid formation from HypF-N under conditions in which the protein is initially in its native state. Journal of Molecular Biology.
2005;
347
(2)
:
323-35
.
View Article PubMed Google Scholar -
Choi
H.S.,
Kim
J.W.,
Cha
Y.N.,
Kim
C.,
A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. Journal of Immunoassay {&}amp; Immunochemistry.
2006;
27
(1)
:
31-44
.
View Article PubMed Google Scholar -
Keller
A.,
Mohamed
A.,
Dröse
S.,
Brandt
U.,
Fleming
I.,
Brandes
R.P.,
Analysis of dichlorodihydrofluorescein and dihydrocalcein as probes for the detection of intracellular reactive oxygen species. Free Radical Research.
2004;
38
(12)
:
1257-67
.
View Article PubMed Google Scholar -
Husain
E.,
Chibber
S.,
Naseem
I.,
Amelioration of 5-FU induced toxicity by using RF under white light: An in vitro study. Integr Pharm Toxicol Genotoxicol.
2015;
1
(1)
:
43-48
.
View Article Google Scholar -
Mojzisová
G.,
Mirossay
L.,
Kucerová
D.,
Kyselovivc
J.,
Miroššay
A.,
Mojvziš
J.,
Protective effect of selected flavonoids on in vitro daunorubicin-induced cardiotoxicity. Phytotherapy Research.
2006;
20
(2)
:
110-4
.
View Article PubMed Google Scholar -
Chinnathambi
S.,
Velmurugan
D.,
Hanagata
N.,
Aruna
P.R.,
Ganesan
S.,
Investigations on the interactions of 5-fluorouracil with bovine serum albumin:Optical spectroscopic and molecular modelling studies. Journal of Luminescence.
2014;
46
(13)
:
1-10
.
View Article Google Scholar -
Sulkowskaa
A.,
Rownickaa
J.,
Bojkoa
B.,
Sulkowski
W.,
Interaction of anticancer drugs with human and bovine serum albumin. Journal of Molecular Structure.
2003;
651
:
133-40
.
View Article Google Scholar -
Hu
X.,
Cui
S.,
Liu
J.,
Fluorescence studies of interaction between flavonol p-coumaroylglucoside tiliroside and bovine serum albumin. Spectrochimica Acta. Part A: Molecular and Biomolecular Spectroscopy.
2010;
77
(2)
:
548-53
.
View Article PubMed Google Scholar -
Bakkialakshmi
S.,
Chandrakala
D.,
A spectroscopic investigations of anticancer drugs binding to bovine serum albumin. Spectrochimica Acta. Part A: Molecular and Biomolecular Spectroscopy.
2012;
88
:
2-9
.
View Article PubMed Google Scholar -
Bannister
W.H.,
Bannister
J.V.,
J. V, Superoxide-dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of `catalytic' iron and anti-oxidant activity in extracellular fluids. The International Journal of Biochemistry.
1974;
5
:
673-7
.
View Article Google Scholar -
Brahms
S.,
Brahms
J.,
Determination of protein secondary structure in solution by vacuum ultraviolet circular dichroism. Journal of Molecular Biology.
1980;
138
(2)
:
149-78
.
View Article PubMed Google Scholar -
Chang
C.T.,
Wu
C.S.,
Yang
J.T.,
Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Analytical Biochemistry.
1978;
91
(1)
:
13-31
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 8 No 12 (2021)
Page No.: 4740-4749
Published on: 2021-12-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4736 times
- PDF downloaded - 1270 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress