Abstract
Stem cell transplantation has the long history of more than 50 years from the first bone marrow transplantation in 1957. From the 2000s, clinical applications of stem cells significantly increased with more diseases and more patients treated with stem cells. Both autologous stem cells and allogenic stem cells as well as adult stem cells and induced pluripotent stem cells (iPSCs), and both in vitro non-expanded stem cells and in vitro expanded stem cells were clinically applied. For adult stem cells, besides hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), neural stem cells, endothelial progenitor cells, limbal stem cells… also were used in the treatment of some diseases. To the year 2015, applications of MSCs have dramatically increased when some MSCs based drugs that were approved and commercialized in some countries. About iPSCs, Japanese scientists also firstly applied the iPSCs in treatment of ophthalmological diseases. Currently, the European Medicines Agency approved the first expanded stem cell therapy to repair damaged cornea in the Europe. This review aimed to summarize, update clinical applications of stem cells to 2015.
Introduction
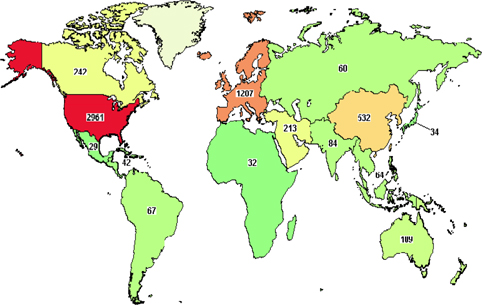
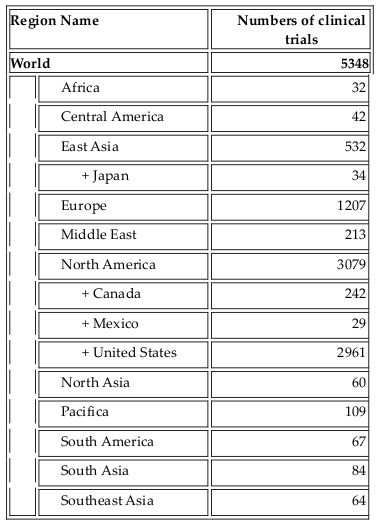
As one of the four bases of healthcare science, stem cell therapy offers advanced treatment for degenerative diseases as well as for some genetic disorders. Initially, bone marrow was used as a source of hematopoietic stem cells. To date, stem cell types which have been used in clinical trials include HSCs, mesenchymal stem cells, neural stem cells, epidermal stem cells, endothelial progenitor cells, limbal stem cells, embryonic stem cells, and induced pluripotent stem cells. Their use in clinical trials strongly increased approximately 10 years ago. As per clinicaltrials.gov, more than 5000 clinical trials use stem cells for treatment of more than 50 different diseases ( Figure 1 , Table 1 ). More importantly, from 2010 onwards, approximately 12 stem-cell based products have been approved for treatments, some of them are regarded as stem cell drugs ( Table 2 ).
Clinical application of HSCs
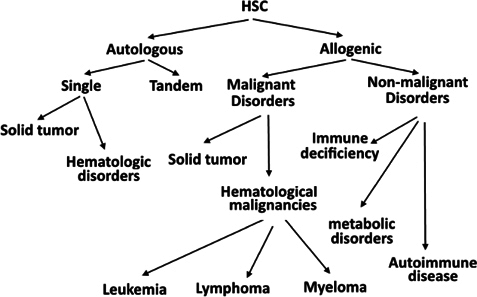
HSC transplantation is now considered to be standard treatment for some types of abnormal hematological conditions, including multiple myeloma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, ß-thalassemia, and sickle cell anemia Giralt et al., 2014. HSCs from bone marrow, peripheral blood, and cord blood are used to clinically treat these hematological diseases Cheuk, 2013. In contrast to HSCs that found in bone marrow(BM) and in umbilical cord blood (UCB), HSCs from peripheral blood were collected after stimulation with cytokines such as granulocyte colony-stimulating factor(G-CSF) to mobilize the HSCs from BM to peripheral blood. In recent studies, HSCs from BM could be mobilized by chemicals used in chemotherapy, called chemomobilization, or by using plerixafor (AMD3100) Giralt et al., 2014 or BIO5192- a small-molecule inhibitor of integrin α4 which interrupts the VCAM-1-VLA-4 (integrin α4) axis Ramirez et al., 2009 ( Figure 2 ). Current clinical trials have also used HSCs for solid tumor treatments and for curing HIV. Although initial results have shown promise, more clinical trials need to be performed to confirm these results before routine therapeutic use.
MSC-based therapies
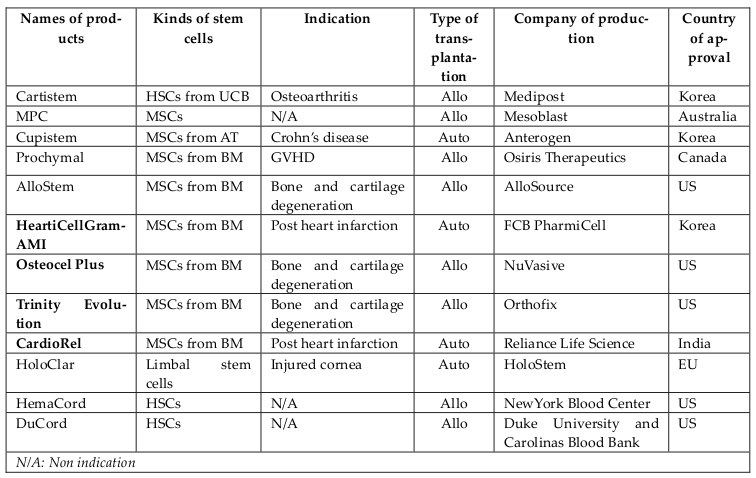
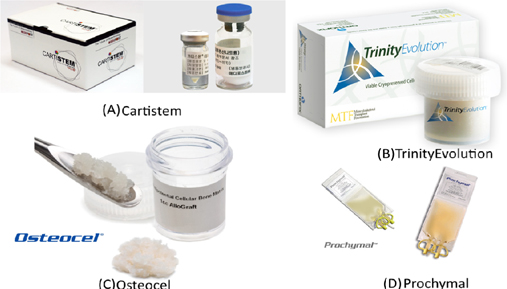
For the past five years, MSC-based therapies have been widely used in clinical applications as one of two main approaches: (1) approved MSC-based products and (2) clinical trials. Some MSC-based products have been approved for clinical applications in several countries for treating diseases involving both autologous and allogeneic transplantation. They have significantly contributed to the growth of MSCs-based therapies and include the following: CARTISTEM® (a combination of human umbilical cord-MSCs (UCMSCs) and sodium hyaluronate for cartilage regeneration), CardioRel® (autologous product designed for early or planned intervention in patients of myocardial infarction), Trinity® Evolution™ (allograft of cancellous bone containing viable adult stem cells and osteoprogenitor cells within the matrix), Osteocel® Plus (allograft cellular bone matrix that retains its native bone-forming cells, including MSCs and osteoprogenitors for the repair, replacement, and reconstruction of skeletal defects), Hearticellgram®-AMI (bone marrow-derived MSCs (BM-MSCs) used to treat acute myocardial infarction (MI) through intracoronary injection), AlloStem (demineralized allograft bone combined with adipose-derived MSCs for general bone grafting applications), and Prochymal (first stem cell drug approved for use in Canada for acute graft-versus-host disease(GVHD) ( Figure 3 ).
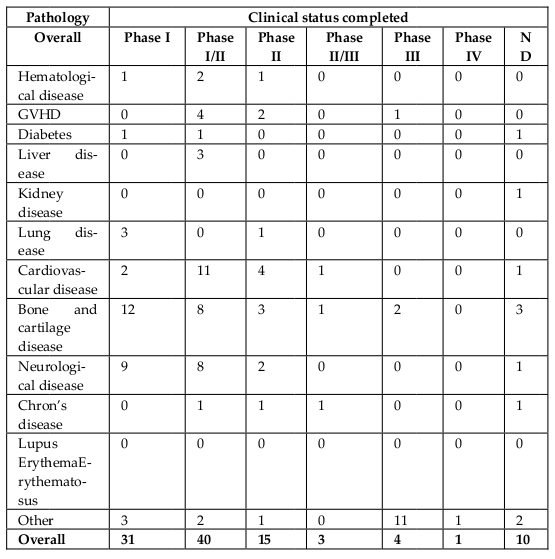
In addition to approved MSCs-based products, MSCs itself have been used in disease treatment through clinical trials. According to clinicaltrials.gov, approximately 542 registered clinical trials have used MSCs for treatment ( Figure 4 , Table 3 ). The first clinical trial using in vitro expanded MSCs was performed in 1995, in which 15 patients were treated with autologous stem cells Lazarus et al., 1995. According to clinicaltrials.gov, almost all current trials are in Phase I, Phase II, or Phase I/II, and some of these trials are in Phase II or Phase II/III. Diseases treated by MSCs-based therapies include hematological diseases, GVHD, diabetes, liver diseases, kidney diseases, lung diseases, cardiovascular diseases, bone and cartilage diseases, neurological diseases, Crohn’s disease, and lupus erythematosus.
Limbal stem cells
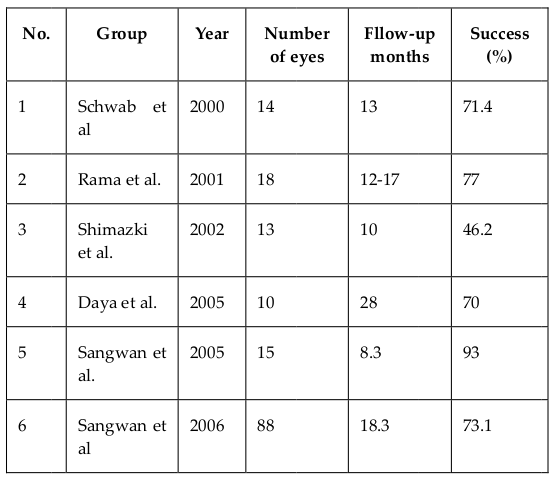
Limbal stem cells (LSCs) have had a long history of clinical applications. LSCs and autograft of limbal tissues were employed by Kenyon and Tseng since 1989 Kenyon and Tseng, 1989. Both fresh limbal tissues Rao et al., 1999Shimazaki et al., 2006Tseng et al., 1998 and cultivated limbal tissues Rama et al., 2001Sangwan et al., 2005aSangwan et al., 2005bTseng et al., 2002 were clinically used. A common procedure was to use limbal tissue collected from healthy eyes and transplant it into injured eyes. However, this procedure was associated with injuries in healthy eyes-donors after the collection of limbal tissue. Recently, allotransplantation, that uses limbal tissue from donors, has been developed. Ang et al. (2007) showed that a limbal allotransplant helped decrease stromal scarring and improved vision in the transplanted eyes. Cultivated limbal epithelial transplantation also significantly improved injury treatment with great success ( Table 4 ). In 2015, the European Commission approvedHoloclar®, the first advanced therapy medicinal product (ATMP) containing LSCs, for clinical use in Europe.
Neural stem cells
Neural stem cells (NSCs) are adult stem cells that can differentiate into glial cells and neurons. Neurological and central nervous system (CNS) disorders include groups of numerous diseases. According to clinicaltrials.gov, approximately 1000 clinical trials tested NSCs for treatments ( Table 5 ). Recently, Phase I and Phase II clinical trials were conducted using NSCs for treatment of CNS disorders. Most of these studies used allogenic NSCs from human fetal tissues. The first clinical trials of NSCs, held in May 2006 at Oregon Health and Science University (OHSU, Portland, OR, USA), involved fetal tissue-derived NSCs for lysosomal storage diseases.
NSCs were also used to treat CNS diseases such as Pelizaeus-Merzbacher disease (PMD), intramedullary spinal cord transplantation of human HuCNS-SC neurospheres in subjects with thoracic (T2-T11) spinal cord trauma, chronic spinal cord injury, Lou Gehrig’s disease, ALS, disabled ischemic stroke, Parkinson’s diseases, Alzheimer’s diseases, and spinal cord injury. To date, there has been a single clinical trial using human ESC-derived neural cells for CNS injury treatment. In 2010, Geron Corporation started Phase I clinical trials using human ESC-derived oligodendrocyte progenitors (GRNOPC1 cells) in patients with neurologically complete, subacute spinal cord injury ( ClinicalTrials.gov identifier:NCT01217008) Lebkowski, 2011Okamura et al., 2007. In 2013, AsteriasBiotherapeutics, Inc., a subsidiary of BioTime, purchased the Geron Corporation’s stem cell division and announced the resumption of its spinal cord trial.
Endothelial progenitor cells
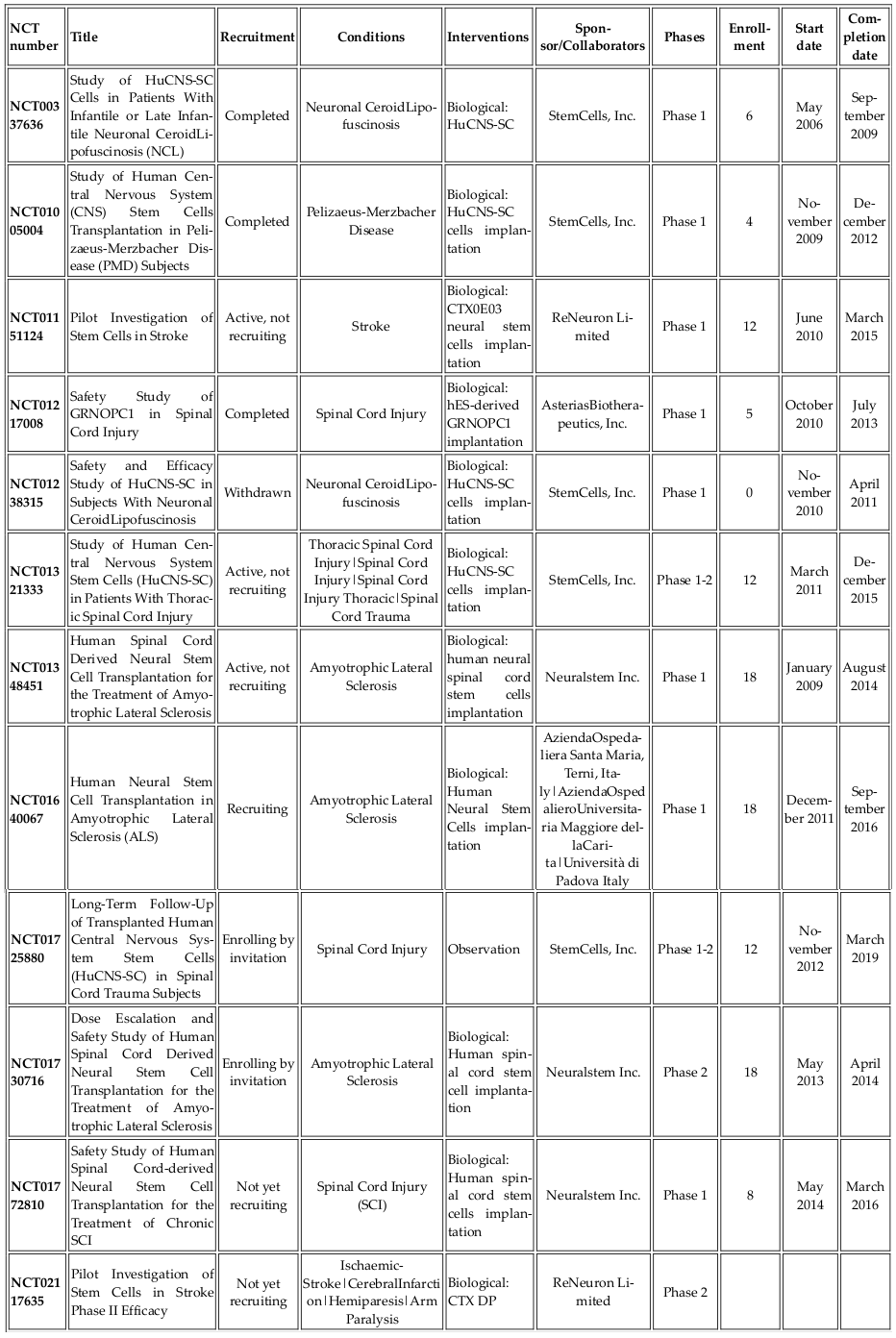
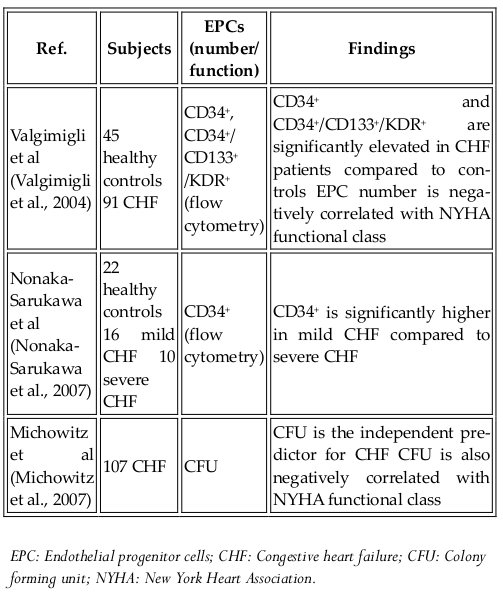
Endothelial progenitor cells (EPCs) were first discovered in 1997. Although their classification as stem cells is controversial, they are considered to be an important source of cells used in regenerative medicine. These cells can differentiate into specific cell types but are limited in self-renewal. EPC-based therapies have been tested in angiogenesis therapy for critical ischemic tissues, post injury vascular endothelial regeneration, and in in vivo tissue engineering. There are two types of EPCs, namely, early EPCs and late EPCs. The early EPCs are spindle-shaped cells that attain peak growth at approximately 2 weeks and die by 4 weeks. Late EPCs appear only as a cobblestone monolayer with near-complete confluence, exhibit exponential population growth without senescence over 4-8 weeks and live for up to 12 weeks. EPCs have been clinically tested for treating diseases Jujo et al., 2008Lee and Poh, 2014Losordo et al., 2007. To date, all clinical trials using EPCs involve autologous transplantation ( Table 6 ). Allogeneic transplantation has been tested in animal models and can be applicable to humans in the near future.
Embryonic stem cells and induced pluripotent stem cells
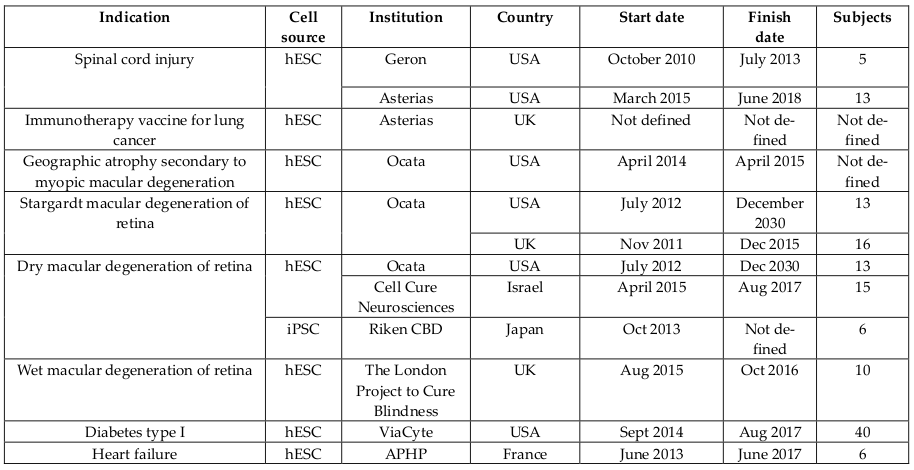
Human embryonic stem cells (ESCs) as well as induced pluripotent stem cells (iPSCs)-based therapies have also moved to clinical trials despite ethical issues. ESCs and IPSCs have been used in two forms, including ESCs and ESCs-derived cells. Human ESCs-derived retinal pigment epithelium was used to treat age-related macular degeneration and Stargardt’s macular dystrophy Schwartz et al., 2015 in Phase I/II ( Table 7 ). Preliminary results after 1 year of treatment showed that visual acuity improved (9-19 letters in three patients) and remained stable (+1 letter in one patient) Song et al., 2015. Besides, ESCs and iPSCs were also used to treat other diseases such as spinal cord injury, geographic atrophy secondary to myopic macular degeneration, Stargardt macular degeneration of retina, dry macular degeneration of retina, wet macular degeneration of retina, diabetes Type I, heart failure, and immunotherapy vaccine for lung cancer. Compared to adult stem cells, ESCs have undergone fewer clinical trials in a limited number of patients but it is expected that they will be widely employed in the near future.
Conclusion
Stem cell therapy has strongly developed since the 2010s to date. Besides hematopoietic stem cell transplantation, mesenchymal stem cell (MSC) transplantation becomes a new choice for regenerative medicine. These initial results from clinical trials of MSC transplantation showed that MSC transplantation is opening a new revolution of stem cell therapy in next decades. Embryonic stem cells as well as pluripotent stem cells have vastpotential for treatment, but they only could become treatment options when the problems about in vivo; in vitro spntaneous differentiation and tumorigenesis in vivo would be strictly controlled. In conclusion, stem cell therapy is continously growing at a high rate, it truly becomes an essential treatment in modern medicine.
References
-
D.K.
Cheuk.
Optimal stem cell source for allogeneic stem cell transplantation for hematological malignancies. World J Transplant.
2013;
3
:
99-112
.
-
S.
Giralt,
L.
Costa,
J.
Schriber,
J.
Dipersio,
R.
Maziarz,
J.
McCarty,
P.
Shaughnessy,
E.
Snyder,
W
Bensinger.
Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant.
2014;
20
:
295-308
.
-
K.
Jujo,
M.
Ii,
D.W.
Losordo.
Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol.
2008;
45
:
530-544
.
-
K.R.
Kenyon,
S.C.
Tseng.
Limbal autograft transplantation for ocular surface disorders. Ophthalmology.
1989;
96
:
709-722; discussion 722
.
-
H.M.
Lazarus,
S.E.
Haynesworth,
S.L.
Gerson,
N.S.
Rosenthal,
A.I.
Caplan.
Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant.
1995;
16
:
557-564
.
-
J.
Lebkowski.
GRNOPC1: the world’s first embryonic stem cell-derived therapy. Interview with Jane Lebkowski. Regen Med.
2011;
6
:
11-13
.
-
P.S.
Lee,
K.K.
Poh.
Endothelial progenitor cells in cardiovascular diseases. World J Stem Cells.
2014;
6
:
355-366
.
-
D.W
Losordo,
C.J.
White,
J.E.
Udelson,
V.
Veereshwarayya,
M.
Durgin,
K.K.
Poh,
R.
Weinstein,
M.
Kearney,
M.
Chaudhry.
Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation.
2007;
115
:
3165-3172
.
-
Y
Michowitz,
D.
Wexler,
D.
Sheps,
G.
Keren,
J.
George.
Circulating endothelial progenitor cells and clinical outcome in patients with congestive heart failure. Heart.
2007;
93
:
1046-1050
.
-
M.
Nonaka-Sarukawa,
K.
Yamamoto,
H.
Aoki,
Y.
Nishimura,
H.
Tomizawa,
M.
Ichida,
T.
Eizawa,
K.
Muroi,
U.
Ikeda,
K.
Shimada.
Circulating endothelial progenitor cells in congestive heart failure. Int J Cardiol.
2007;
119
:
344-348
.
-
R.M.
Okamura,
J.
Lebkowski,
M.
Au,
C.A.
Priest,
J.
Denham,
A.S.
Majumdar.
Immunological properties of human embryonic stem cell-derived oligodendrocyte progenitor cells. J Neuroimmunol.
2007;
192
:
134-144
.
-
P.
Rama,
S.
Bonini,
A.
Lambiase,
O.
Golisano,
P
Paterna,
G.
Pellegrini.
Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency!. Transplantation.
2001;
72
:
1478-1485
.
-
P.
Ramirez,
M.P.
Rettig,
G.L.
Uy,
E.
Deych,
M.S.
Holt,
J.K.
Ritchey,
J.F.
DiPersio.
BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood.
2009;
114
:
1340-1343
.
-
S.K.
Rao,
R.
Rajagopal,
G.
Sitalakshmi,
P
Padmanabhan.
Limbal autografting: comparison of results in the acute and chronic phases of ocular surface burns. Cornea.
1999;
18
:
164-171
.
-
V.S.
Sangwan,
H.P.
Matalia,
G.K.
Vemuganti,
G.
Ifthekar,
A.
Fatima,
S.
Singh,
G.N.
Rao.
Early results of penetrating keratoplasty after cultivated limbal epithelium transplantation. Archives of ophthalmology.
2005a;
123
:
334-340
.
-
V.S.
Sangwan,
B.
Ramamurthy,
U.
Shah,
P.
Garg,
M.S.
Sridhar,
G.N.
Rao.
Outcome of corneal transplant rejection: a 10-year study. Clinical & experimental ophthalmology.
2005b;
33
:
623-627
.
-
S.D.
Schwartz,
C.D.
Regillo,
B.L.
Lam,
D.
Eliott,
P.J.
Rosenfeld,
N.Z.
Gregori,
J.P.
Hubschman,
J.L.
Davis,
G.
Heilwell,
M.
Spirn.
Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet.
2015;
385
:
509-516
.
-
J.
Shimazaki,
K.
Konomi,
S.
Shimmura,
K.
Tsubota.
Ocular surface reconstruction for thermal burns caused by fireworks. Cornea.
2006;
25
:
139-145
.
-
W.K.
Song,
K.M.
Park,
H.J.
Kim,
J.H.
Lee,
J.
Choi,
S.Y.
Chong,
S.H.
Shim,
L.V
Del Priore.
Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports.
2015;
4
:
860-872
.
-
S.C.
Tseng,
D.
Meller,
D.F.
Anderson,
A.
Touhami,
R.T.
Pires,
M.
Grüterich,
A.
Solomon,
E.
Espana,
H.
Sandoval,
S.-E.
Ti.
Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane for treating corneal diseases with total limbal stem cell deficiency. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3 (Springer).
2002;
:
1323-1334
.
-
S.C.
Tseng,
P.
Prabhasawat,
K.
Barton,
T.
Gray,
D.
Meller.
Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Archives of Ophthalmology.
1998;
116
:
431-441
.
-
M.
Valgimigli,
G.M.
Rigolin,
A.
Fucili,
M.D.
Porta,
O.
Soukhomovskaia,
P.
Malagutti,
A.M.
Bugli,
L.Z.
Bragotti,
G.
Francolini,
E.
Mauro.
CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation.
2004;
110
:
1209-1212
.
Comments

Downloads
Article Details
Volume & Issue : Vol 3 No 02 (2016)
Page No.: 483-489
Published on: 2016-02-17
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 15263 times
- Download PDF downloaded - 2132 times
- View Article downloaded - 12 times
 Biomedpress
Biomedpress