Abstract
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is a hereditary enzymatic disorder that can result in acute hemolytic anemia under certain conditions. Favism, induced by ingesting fava beans, is a well-known trigger of hemolysis in individuals with G6PD deficiency. We report the case of a five-year-old girl who presented to our hospital with signs and symptoms of hemolysis. At admission, we were unaware that she had an underlying G6PD deficiency and detected it while performing laboratory investigations to determine the etiology.
Introduction
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common hereditary disorder, affecting about 400 million individuals worldwide1. It is characterized by reduced G6PD activity in red cells and hemolysis, usually after exposure to oxidant stress. Because G6PD deficiency is an X-linked recessive disorder, it clinically manifests primarily in males, while its expression varies in heterozygous females2. The first case of G6PD deficiency was reported in 1956 when an individual experienced hemolysis after taking the antimalarial drug primaquine. Hemolysis in individuals with G6PD deficiency has since been associated with various infections, fava bean consumption, and certain oxidative medications3. Here, we report a five-year-old girl who presented with severe hemolytic anemia secondary to fava bean ingestion.
Case Report
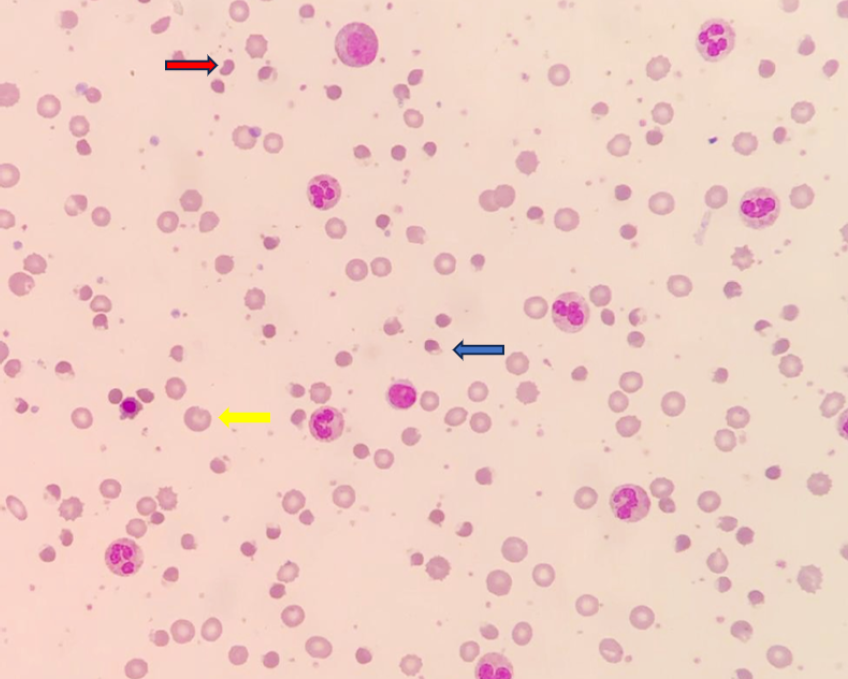
A five-year-old Malay girl presented with a history of fever for three days, anemic symptoms, and tea-colored urine. During her physical examination, she appeared pallor, jaundiced, and tachycardic, with a heart rate of 110 bpm. Her other vital signs were unremarkable. Laboratory investigations showed low hemoglobin (5.9 g/dL) and a high reticulocyte count (8.29%). Her indirect bilirubin level was 110 umol/L, and serum lactate dehydrogenase was 270 U/L. A peripheral blood film examination showed normocytic normochromic red cells, many blister cells, some polychromatic cells, irregularly contracted cells, and a few bite cells.
She was initially treated for infection-induced hemolysis since a history of G6PD deficiency had not been disclosed. On further questioning, her mother claimed that she had underlying G6PD deficiency, which had been diagnosed at birth, and before admission, she had been eating fava beans. She was treated with two units of pack red cell transfusions, and her hemoglobin was 10 g/dL post-transfusion. She was discharged a few days later.
Discussion
G6PD deficiency is the most common X-linked genetic disorder. Since males have a single X chromosome, they have either a G6PD-deficient or G6PD-normal genotype. Since females have two X chromosomes, they can be homozygous or heterozygous for G6PD alleles. Heterozygous females carry one chromosome encoding a G6PD enzyme with normal activity and another encoding a G6PD enzyme with deficient activity4. Due to X-inactivation, also known as lyonization, heterozygous females show genetic mosaicism, resulting in a greater variation in phenotypic expression. Our patient was female, so her genotype may be homozygous or heterozygous.
Favism, a severe hemolytic anemia triggered by consuming fava beans, has long been associated with G6PD deficiency5. In this case, the causative agents are two components found in fava beans: vicine and convicine. These components induce the production of free radicals, leading to glutathione oxidation. G6PD deficiency results in a decreased level of NADPH, which is crucial for reducing oxidized glutathione, particularly in red blood cells. The oxidized glutathione acts as a potent oxidizing agent, ultimately causing the onset of a hemolytic crisis6. Hemolytic crisis caused by fava beans is rare in children with G6PD deficiency. A similar case has been reported in Thailand7, but the frequency of fava bean-induced hemolytic crisis in Malaysia is unknown.
In children with G6PD deficiency, the clinical manifestations of hemolytic anemia can include fatigue, irritability, and pallor. Red cell lysis can lead to shortness of breath and tachycardia due to decreased oxygen levels. Additional symptoms may include fever, darkened urine, lower back pain, abdominal discomfort, and an enlarged spleen. Hemolytic anemia due to favism, which is more common and severe in children, might manifest about 1–2 days after consuming trigger substances. It could be accompanied by a mild fever and either lethargy or irritability8.
Good history taking can attain the diagnosis of acute hemolysis secondary to favism. No extensive laboratory investigations are needed. Only basic laboratory findings characterized by anemia with reticulocytosis, high level of indirect hyperbilirubinemia, elevated LDH, and typical red cell morphology oxidative features are the clues to the diagnosis. The qualitative screening of the G6PD enzyme through a G6PD fluorescent spot test demonstrates high sensitivity and a high negative predictive value. Nevertheless, G6PD levels can be misleadingly normal during acute hemolytic episodes due to a higher G6PD enzyme activity in reticulocytes than in mature red cells9. Therefore, interpreting G6PD results during such episodes should be approached with caution. This test was not recommended in our case since it may produce false positive results.
Treatment during a hemolytic attack is supportive. A blood transfusion may be indicated in severe cases10. Adequate urinary output should be maintained to prevent renal damage due to hemoglobinuria. As for prevention, patients should be advised to avoid oxidant drugs that can precipitate hemolysis11.
Conclusions
Adequate and relevant clinical history is the leading factor for an accurate diagnosis. Therefore, unnecessary laboratory investigations can be avoided, and the management of the patient can be tailored appropriately. In our case, the underlying history of G6PD deficiency had been missed since she was a girl, and this condition rarely presents in females. Detecting underlying causes in acute crises is important for patient management and preventing requests for unnecessary high-profile investigations.
Abbreviations
G6PD: Glucose-6-phosphate dehydrogenase
Acknowledgments
None.
Author’s contributions
NR, NANA and SA are responsible for the writing of the article. JHHJ and LPC helped in data collection and interpretation. AD and SMAR participated in sequence alignment. All authors read and approved the final manuscript.
Funding
None
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Shen
S.,
Xiong
Q.,
Cai
W.,
Xiong
H.,
Hu
X.,
A novel G6PD gene variant in a Chinese girl with favism. Journal of Clinical Laboratory Analysis.
2020;
34
(9)
:
e23402
.
View Article PubMed Google Scholar -
Frelick
R.W.,
Benge
J.H.,
Favism; a case report. Delaware Medical Journal.
1955;
27
(4)
:
73-5
.
View Article PubMed Google Scholar -
Francis
R.O.,
Jhang
J.S.,
Pham
H.P.,
Hod
E.A.,
Zimring
J.C.,
Spitalnik
S.L.,
Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sanguinis.
2013;
105
(4)
:
271-82
.
View Article PubMed Google Scholar -
Domingo
G.J.,
Advani
N.,
Satyagraha
A.W.,
Sibley
C.H.,
Rowley
E.,
Kalnoky
M.,
Addressing the gender-knowledge gap in glucose-6-phosphate dehydrogenase deficiency: challenges and opportunities. International Health.
2019;
11
(1)
:
7-14
.
View Article PubMed Google Scholar -
Ahmed
S.N.,
Do favic patients resume fava beans ingestion later in their life, a study for this, and a new hypothesis for favism etiology. Hematology/Oncology and Stem Cell Therapy.
2013;
6
(1)
:
9-13
.
View Article Google Scholar -
Beretta
A.,
Manuelli
M.,
Cena
H.,
Favism: Clinical Features at Different Ages. Nutrients.
2023;
15
(2)
:
343
.
View Article PubMed Google Scholar -
Lertthammakiat
S.,
Itdhi-Amornkulchai
S.,
Favism‐induced methemoglobinemia in a G6PD deficient male with a subsequent hemolytic cascade, a therapeutic challenge: Case report and review of literature. Clinical Case Reports.
2021;
9
(4)
:
2048-52
.
-
Harcke
S.J.,
Rizzolo
D.,
Harcke
H.T.,
G6PD deficiency: an update. JAAPA: Official Journal of the American Academy of Physician Assistants.
2019;
32
(11)
:
21-6
.
View Article PubMed Google Scholar -
Roper
D.,
Layton
M.,
Rees
D.,
Lambert
C.,
Vulliamy
T.,
De la Salle
B.,
British Society for Haematology
Laboratory diagnosis of G6PD deficiency. A British Society for Haematology Guideline. British Journal of Haematology.
2020;
189
(1)
:
24-38
.
View Article PubMed Google Scholar -
Bubp
J.,
Jen
M.,
Matuszewski
K.,
Caring for Glucose-6-Phosphate Dehydrogenase (G6PD)-Deficient Patients: implications for Pharmacy. Pharmacy and Therapeutics.
2015;
40
(9)
:
572-4
.
PubMed Google Scholar -
Karyana
I.,
Mudita
I.,
Fava bean-induced hemolytic crisis in glucose-6-phosphate dehydrogenase deficiency. Paediatrica Indonesiana.
2016;
43
(6)
:
230
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 10 (2023)
Page No.: 5953-5955
Published on: 2023-10-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4974 times
- PDF downloaded - 1316 times
- XML downloaded - 90 times
 Biomedpress
Biomedpress



