Docetaxel and a novel bcl-2 antisense oligonucleotide for enhanced apoptosis in prostate cancer cells: A potential therapeutic strategy
- Department of Medical Genetics, School of Advanced Technologies in Medicine, Golestan University of Medical Sciences, Gorgan, Iran
- Cancer Research Center, Golestan University of Medical Sciences, Gorgan, Iran
- Research Center for Stem Cell, Golestan University of Medical Sciences, Department of Medical Genetics, School of Advanced Technologies in Medicine, Golestan University of Medical Sciences, Gorgan, Iran
- Department of Medical Biochemistry, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- Proteomics Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran
- Department of Molecular Genetics, Institute of Basic Science, Shahrekord Islamic Azad University, Iran
Abstract
Background: Prostate cancer is a leading cause of morbidity and mortality among men. Overexpression of Bcl-2 proteins is linked to prostate cancer progression, suggesting that targeting Bcl-2 could enhance therapeutic efficacy. Therefore, Bcl-2 inhibition may potentiate anti-tumor activity in prostate cancer.
Methods: We designed a novel antisense oligonucleotide (ASO) derived from G3139 and evaluated its anti-proliferative effects in LNCaP and PC3 prostate cancer cell lines. The apoptotic effects of docetaxel (DTX) and the ASO, administered alone or in combination, were assessed by real-time PCR and Annexin V-FITC/PI flow cytometry. We hypothesized that the combination therapy would be more effective than either agent alone.
Results: The ASO significantly down-regulated Bcl-2 mRNA and inhibited proliferation in both cell lines, as shown by real-time PCR. Flow cytometry revealed an increase in early apoptotic cells in all treated groups. Co-treatment with the ASO and DTX produced the highest apoptotic rates (75–85 %).
Conclusions: The novel ASO effectively reduces Bcl-2 expression and sensitizes prostate cancer cells to docetaxel, permitting lower DTX doses. The combination therapy induced 75–85 % apoptosis in LNCaP and PC3 cells, suggesting an improved strategy that may limit high-dose DTX–associated resistance and toxicity.
Introduction
Prostate cancer is the second most common cancer and the fifth leading cause of cancer-related death in men. The incidence and mortality rates of prostate cancer are strongly related to age, with the highest rates observed in men over 65 years. Other risk factors include ethnicity, genetics, and family history1, 2, 3, 4. The progression and development of prostate cancer is largely related to the number of genetic abnormalities that affect not only the androgen receptor but also the regulation of apoptotic pathways5. Prostate cancer growth is initially androgen-dependent, but later becomes androgen-independent and is accompanied by increased expression of anti-apoptotic genes, leading to metastasis and higher mortality. Studies show that altered expression of apoptosis-regulating proteins contributes to therapy-resistant prostate cancer. In addition, defects in apoptotic pathways promote cancer-cell survival and resistance to chemotherapy drugs6, 7, 8, 9.
The BCL-2 family plays an essential role in the regulation of cellular apoptosis. BCL-2 is a key anti-apoptotic gene in this pathway10. The BCL-2 protein is anti-apoptotic, and its expression is directly related to the progression of many cancers. Overexpression of this gene is associated with resistance to several therapeutic stimuli, including androgen deprivation, chemotherapy, and radiotherapy11, 12, 13, 14. Overexpression of the BCL-2 protein, common in malignant cells, promotes survival and drug resistance but also presents opportunities for targeted therapies that selectively eliminate these cells15. Thus, combining BCL-2 inhibition with other treatments may enhance cancer-cell killing and limit drug resistance and metastasis.
A common way to treat prostate cancer is chemotherapy; docetaxel (DTX) is often referred to as the first-line treatment. It stabilizes microtubules, thereby inducing cancer-cell death16, 17. Another approach involves antisense oligonucleotides (ASOs), used alone or in combination with other agents. ASOs are chemically modified single-stranded oligodeoxynucleotides of 18–21 nt. These ASOs hybridize to their specific mRNAs, and the RNase H complex subsequently degrades the duplex. In other words, such ASOs can prevent translation of the target gene by binding to the mRNA18, 19, 20. Oblimersen (G-3139; Genasense; Genta Inc.) is an ASO that inhibits translation of BCL-221.
The use of antisense oligonucleotides targeting BCL-2 in combination with docetaxel reduced the IC₅₀ of DTX compared with DTX alone. This reduction helps minimize toxicity to normal cells22. Furthermore, the combination therapy sensitized cancer cells to DTX and decreased their drug resistance22, 23. As high doses of chemotherapy agents typically induce resistance in cancer cells, diminishing the dose can be a strategy to overcome drug resistance effectively22.
We designed a novel antisense oligonucleotide, based on the Ensembl BCL-2 mRNA sequence and the G3139 antisense oligonucleotide, to investigate its apoptotic effects on LNCaP and PC3 prostate-cancer cell lines, alone and in combination with docetaxel. We assessed apoptosis by real-time RT-PCR and flow cytometry, using Lipofectamine for delivery of docetaxel and the novel ASO. We hypothesized that targeting BCL-2 with ASO would enhance docetaxel-induced apoptosis by reducing the IC₅₀ of DTX.
Studies have shown that combining low-dose docetaxel with chemosensitizers can induce effective cytotoxicity in prostate-cancer cells while sparing normal prostate epithelial cells24. Clinically, reduced-dose docetaxel regimens have also demonstrated comparable efficacy with significantly decreased systemic toxicity25. In a rat study, histological examination of vital organs, including the prostate, two weeks after ASO treatment revealed no toxicity26.
Methods
In this study, we designed a novel 22-mer antisense oligonucleotide (ASO) based on the Ensembl BCL-2 mRNA sequence and the previously reported 18-mer ASO G3139. We then evaluated and compared the pro-apoptotic effects of docetaxel (DTX) and the new ASO in LNCaP and PC3 prostate cancer cell lines using real-time PCR and flow cytometry.
Cell Culture
Prostate cancer cell lines LNCaP and PC3 were purchased from the Pasteur Institute (Tehran, Iran). Cells were cultured in RPMI-1640 supplemented with 10 % FBS and maintained at 37 °C in 5 % CO. Cells were passaged at ~95 % confluence; medium was renewed every doubling time. The doubling times for LNCaP and PC3 were 72 h and 48 h, respectively.
Docetaxel (DTX) Preparation
Docetaxel (DTX) was obtained from Actover, dissolved in DMSO to 4 mg mL⁻¹ (10 mg in 2.5 mL) according to the manufacturer’s instructions, aliquoted, and stored at −20 °C.
Antisense Oligonucleotide Design and Preparation
G3139 (oblimersen sodium, Genasense) is an 18-mer antisense oligonucleotides (5′–TCT CCC AGC GTG CGC CAT–3′) complementary to codons 1–6 of the Bcl-2 mRNA27. Novel antisense oligonucleotides (ASO) designed according to the published nucleotide sequence of the bcl-2 mRNA in the Ensemble database (https://asia.ensembl.org/Homo_sapiens) and based on the antisense oligonucleotide G3139.
The secondary structure of the bcl-2 mRNA predicated by minimum free energy (MFE) approach (http://rna.tbi.univie.ac.at//cgibin/RNAWebSuite/RNAfold.cgi). The sequence of ASO was 5ˊ-GTTCTCCCAGCGTGCGCCATCC-3ˊ. To enhance the stability of the antisense oligonucleotide at the target site within cells, we designed it with phosphorothioate modifications at both ends. It has 22 nucleotides, all 22 nucleotides have phosphorthioate modification but the first six nucleotides and the last six nucleotides also have 2’-O-(2-methoxyethyl) modification. It Purchased from Gorgon Gene Inhibition Biotechnology Company (Iran) (
The secondary structure of the bcl-2 mRNA address on minimum free energy (MFE) access with (http://rna.tbi.univie.ac.at//cgibin/RNAWebSuite/RNAfold.cgi) can exhibit in Figure 1.
Bcl-2 antisense oligonucleotide specifications
|
Sequence: 5'- *G2 -*T2-*T2-*C2-*T2-*C2-*C-*C-*A-*G-*C-*G-*T-*G-*C-*G-*C2-*C2-*A2-*T2-*C2-*C2 -3' | |
|
Concentration: 100 µM |
Synthesis scale: 0.20 µmol |
|
GC%:68.2 |
MW: 6977 g/mol |
|
Delivered: 349 µg-50.1 nmol |
Synthesised: 10.5 OD |
|
Quality Control: MALDI-TOF |
Purification: HPLC |
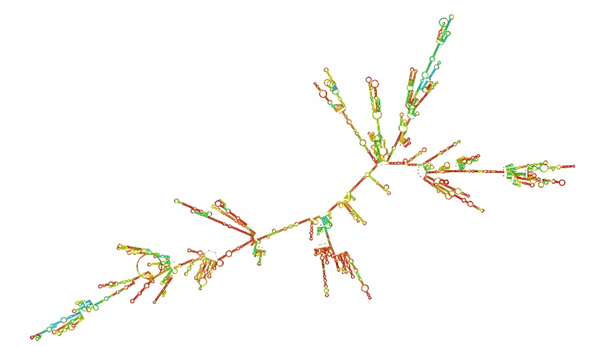
Prediction of secondary structure of mRNA bcl-2 based on MFE.
Binding Analysis
The binding energies of the novel ASO and G3139 were compared . A more negative ΔG indicates higher duplex stability. G3139 displayed a ΔG of −27.53 kcal mol⁻¹ (Figure 2B), whereas the novel ASO reached −36.77 kcal mol⁻¹ (Figure 2A), suggesting ~9 kcal mol⁻¹ stronger binding to mRNA.
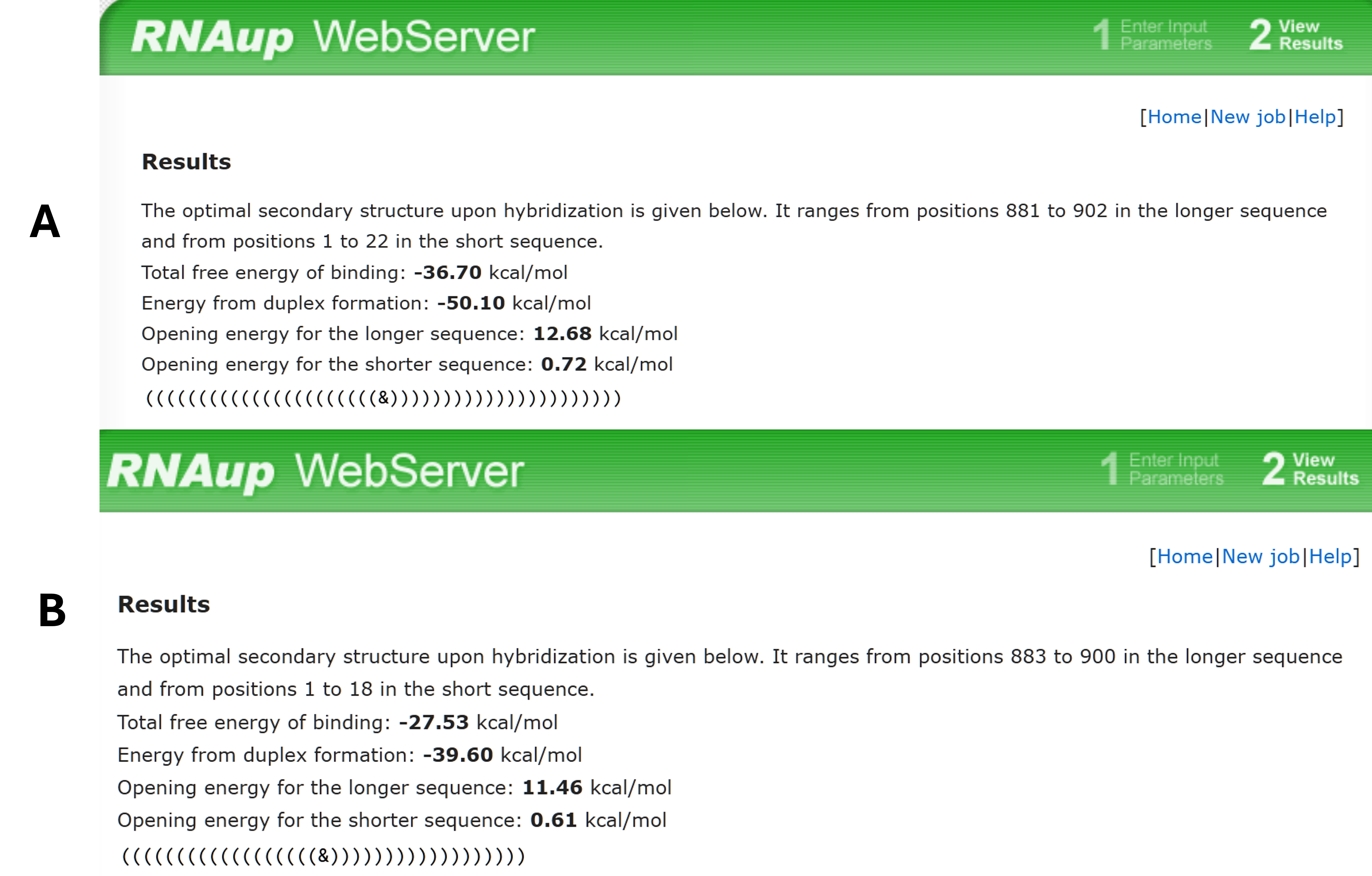
The bioinformatic approach was employed using [ http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAup.cgi ] to predict ΔG of hybridization between the designed ASO and the target mRNA (A). Also between G3139 and target mRNA (B).
Primer Design for Real-Time PCR
Real-time PCR primers were designed with Primer3 and Oligo 7 from the RefSeq sequences of GAPDH and BCL-2 (https://ncbi.nlm.nih.gov/RefSeq). GAPDH: forward 5′-CCT GCC GTC TAG AAA AAC CTG CCA-3′, reverse 5′-CAG CGT CAA AGG TGG AGG AGT GGG-3′ (156 bp). BCL-2: forward 5′-GAC GAC TTC TCC CGC CGC TAC-3′, reverse 5′-TCC CCC AGT TCA CCC CGT CC-3′ (133 bp). Primer-BLAST confirmed specificity (Figure 3).
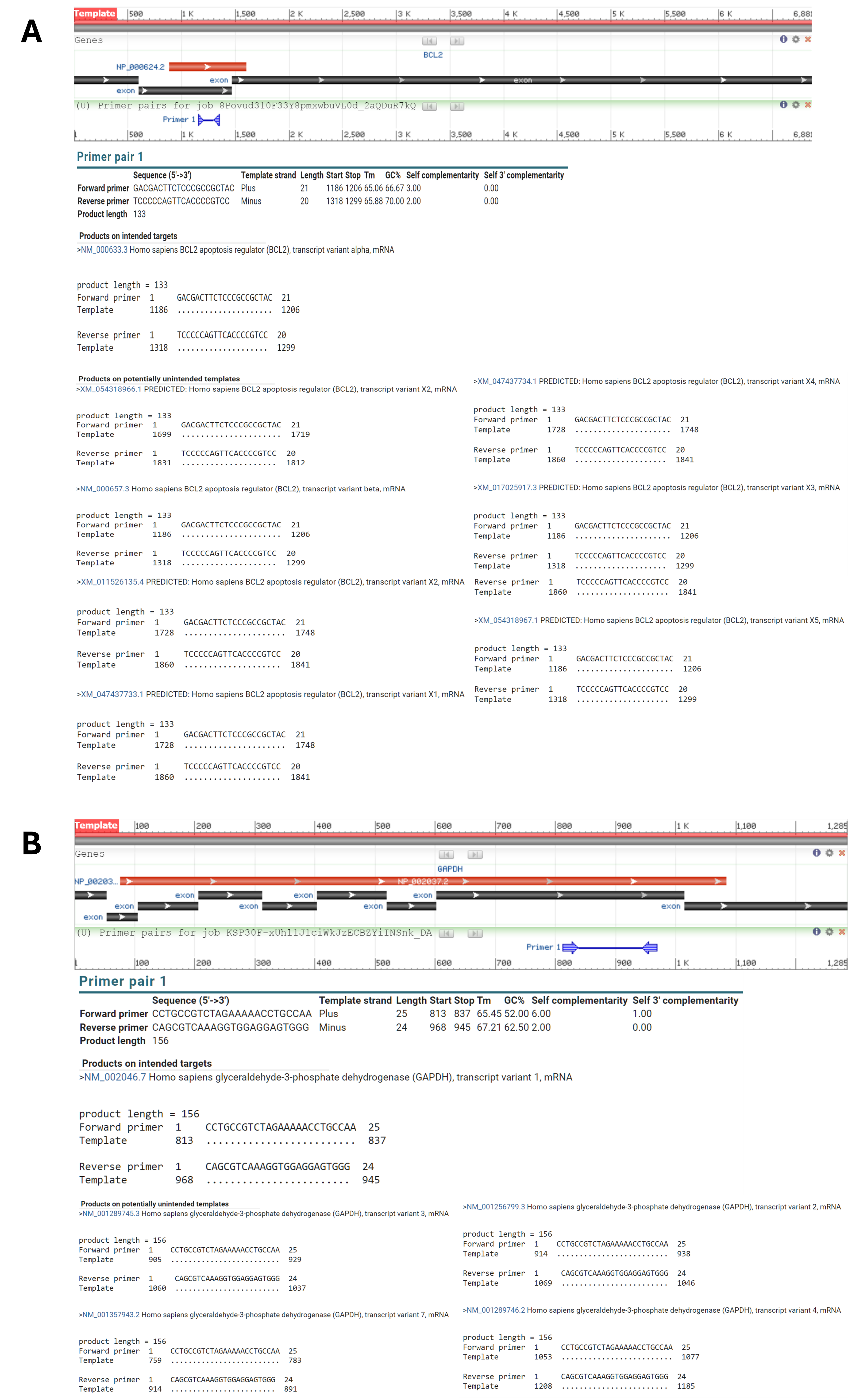
Result of primer blast for A)
Transfection and treatment of two cell lines with Antisense Oligonucleotide design and DTX
|
Cell lines |
Control (48 and 72h) |
Docetaxel (DTX) (48 and 72h) |
New designed ASO (48 and 72h) |
Docetaxel (DTX) and new ASO encapsulated with lipofectamine (48 and 72h) |
|
LNCaP |
* |
* |
* |
* |
|
PC3 |
* |
* |
* |
* |
Transfection of Cells
LNCaP and PC3 cells at passage 6 were transfected with the ASO using Lipofectamine 2000 (Invitrogen) at a 1:1 (v/v) ratio (50 µL each) in serum- and antibiotic-free medium (
RNA Isolation and cDNA Synthesis
Total RNA was isolated 48 h and 72 h post-transfection using TRIzol (Invitrogen) followed by DNase I treatment (Yekta Tajhiz, Iran). Quantity and purity were assessed spectrophotometrically (A₂₆₀/₂₈₀) and by agarose-gel electrophoresis, then verified with the BIOFACT RP101-050 kit. First-strand cDNA was synthesized with the YTA cDNA synthesis kit (Yekta Tajhiz).
Analysis of BCL-2 mRNA Expression
Real-time PCR reactions (12 µL) contained 1–2 µL cDNA, 0.5 µL each primer (5 pmol), 5 µL RealQ Plus SYBR Green master mix (Amplicon, Denmark), and 4–5 µL nuclease-free water. Cycling conditions were 95 °C/10 min; 45 cycles of 95 °C/30 s, 60 °C/30 s, 72 °C/30 s on an ABI StepOne system. Amplification efficiency (E = 10) was calculated from standard curves (Figure 4). Relative expression was determined by ΔΔ using GAPDH as the reference.
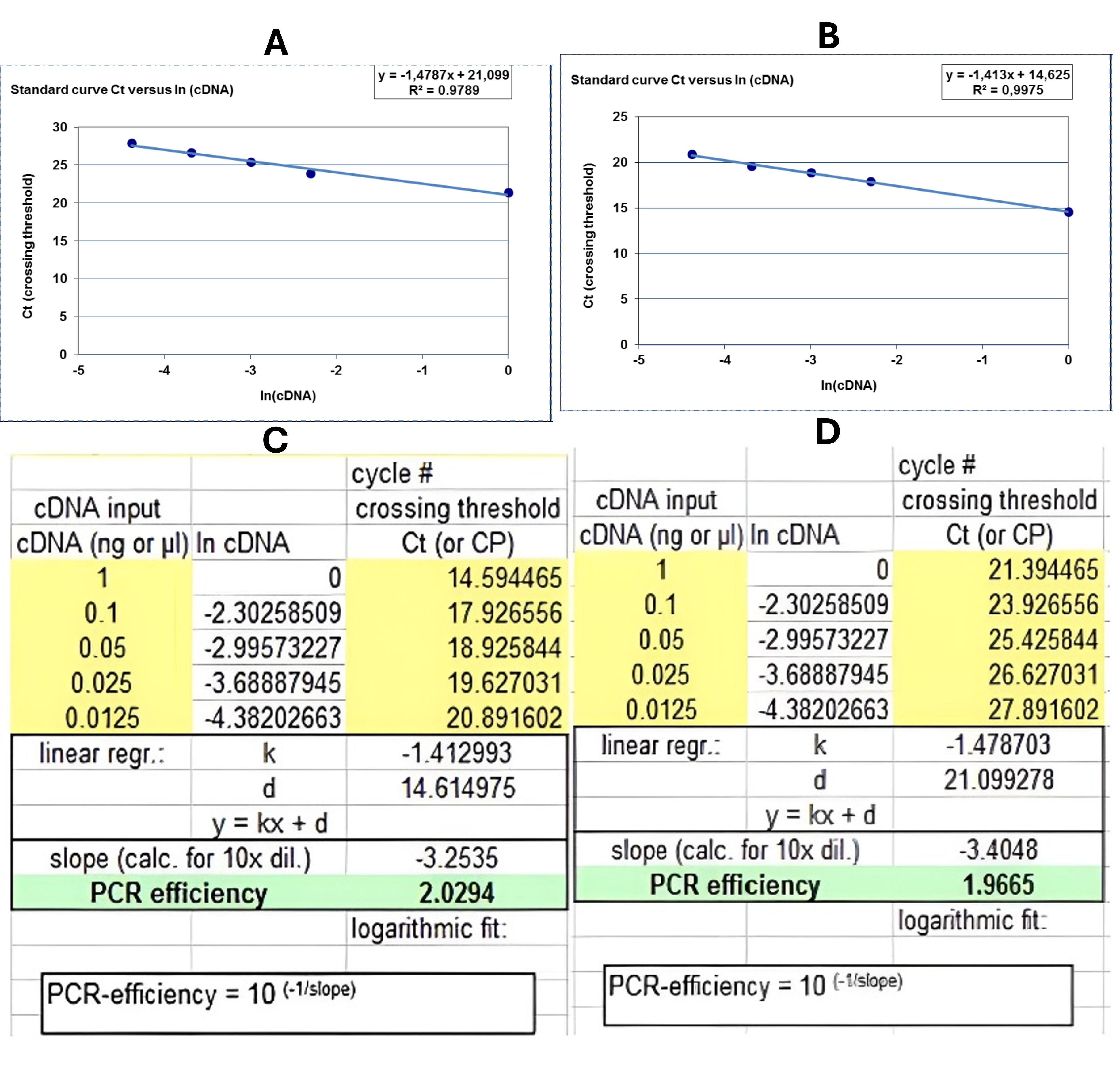
Result of primer efficacy based on standard curves adapted from PCR. A)
Evaluation of Cell Cytotoxicity by MTT Assay
LNCaP and PC3 cells (5 × 10³ cells/200 µL) were seeded in 96-well plates. DTX cytotoxicity was measured by MTT assay after 48 h and 72 h. Concentration ranges were 2–400 nM (LNCaP) and 10–800 nM (PC3). IC₅₀ values guided combination studies in which cells were first transfected with 125 or 250 nM ASO for 5 h, then treated with DTX at or below the IC₅₀. Absorbance at 570 nm was recorded. All experiments were performed in triplicate.
Determination of Apoptosis by Flow Cytometry
Apoptosis was quantified with the Annexin V-FITC/PI Kit (MabTag, Germany) according to the manufacturer’s protocol. Cells were harvested after treatments defined by the MTT and qRT-PCR results, stained, and analyzed on a BD FACSArray with FlowJo software. Each condition was tested in triplicate.
Statistical Analysis
Data are presented as mean ± SEM (n = 3). One-way ANOVA followed by Student’s t-test was used to assess significance. Graphs were generated with GraphPad Prism 8. PCR data were analyzed with LinRegPCR 11.0 and REST. Drug-interaction synergy was calculated by the Bliss independence model (
Results
Real-Time RT-PCR
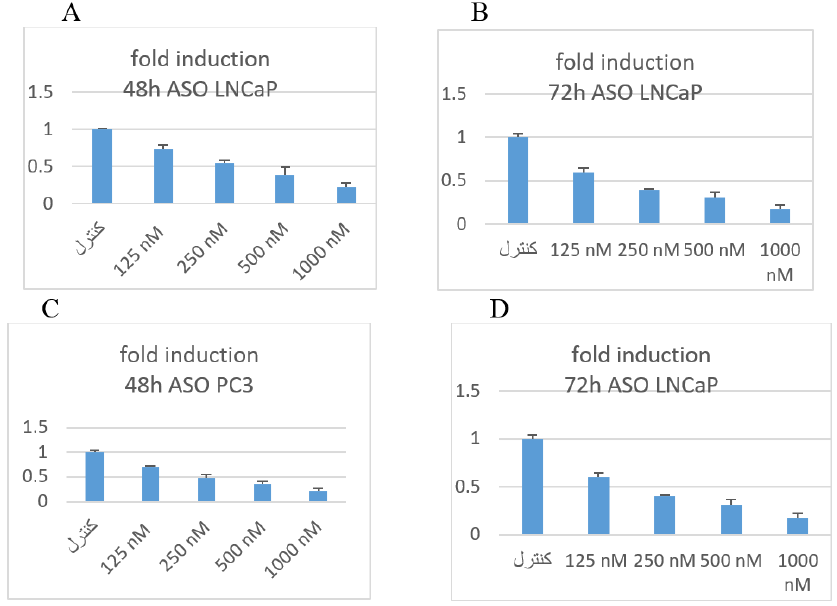
To evaluate the effect of the newly designed ASO on BCL-2 expression in two prostate-cancer cell lines, we performed real-time RT-PCR as described in the Transfection section. BCL-2 mRNA levels were normalized to the housekeeping gene GAPDH. Down-regulation of BCL-2 was observed at both 48 h and 72 h with ASO concentrations of 250 nM and 125 nM, respectively (Figure 5).
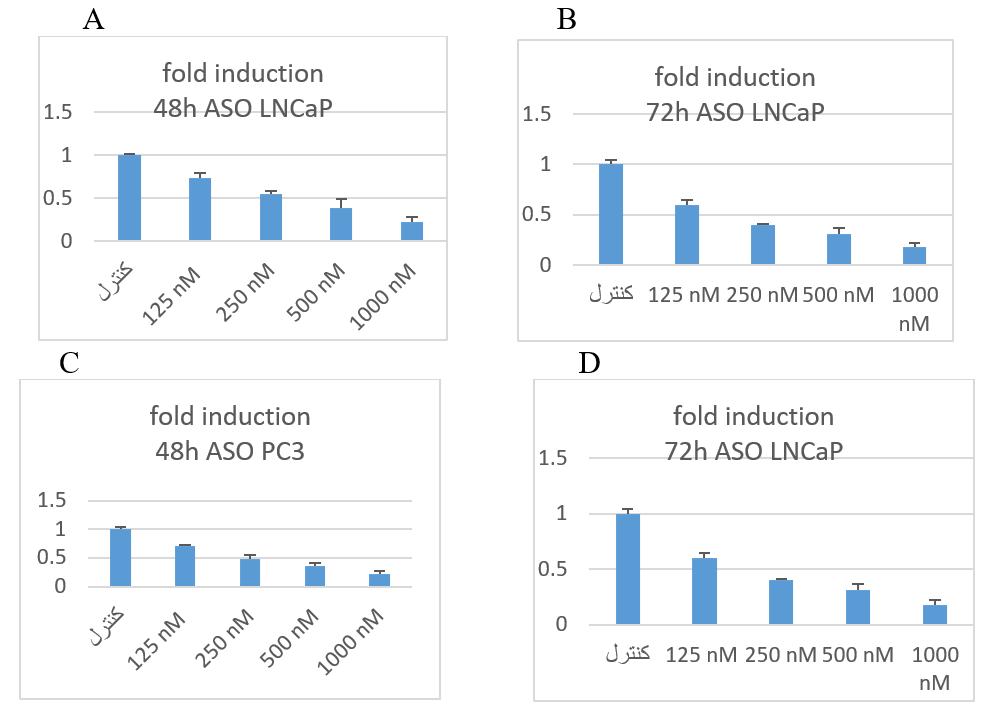
The result of fold change of real time PCR. In this method ΔΔCT was applied. A and B was related to LNCaP cell lines. C and D was related to PC3.
MTT Assay
Cell viability decreased in a time- and dose-dependent manner. In the DTX-only group, after 48 h and 72 h, the IC values were 135 nM and 63 nM for LNCaP cells and 89 nM and 57 nM for PC3 cells (Figure 6A–D). In the ASO-only group, the IC values for LNCaP cells were 273 nM (48 h) and 189 nM (72 h), whereas for PC3 cells they were 309 nM (48 h) and 188 nM (72 h) (Figure 6E,F). In the ASO + DTX group, the IC values for LNCaP cells were 75 nM (48 h) and 32 nM (72 h) (Figure 6G,H); for PC3 cells they were 48 nM (48 h) and 32 nM (72 h) (Figure 6I,J).
In PC3 cell line in 48 h in the control group, bcl-2 relative to the GAPDH was normal (100%). Nevertheless, in cells that had taken 125 nM, the expression of the bcl-2 in relation to the GAPDH was almost 70%. In cells which had 500 nM ASO, the expression of bcl-2 mRNA in ratio of GAPDH was approx 49%. The cells that had taken 500 nM, the expression of the bcl-2 in relation to the GAPDH was almost 36%. In cells that had taken 1000 nM ASO, the expression of bcl-2 was 21%.
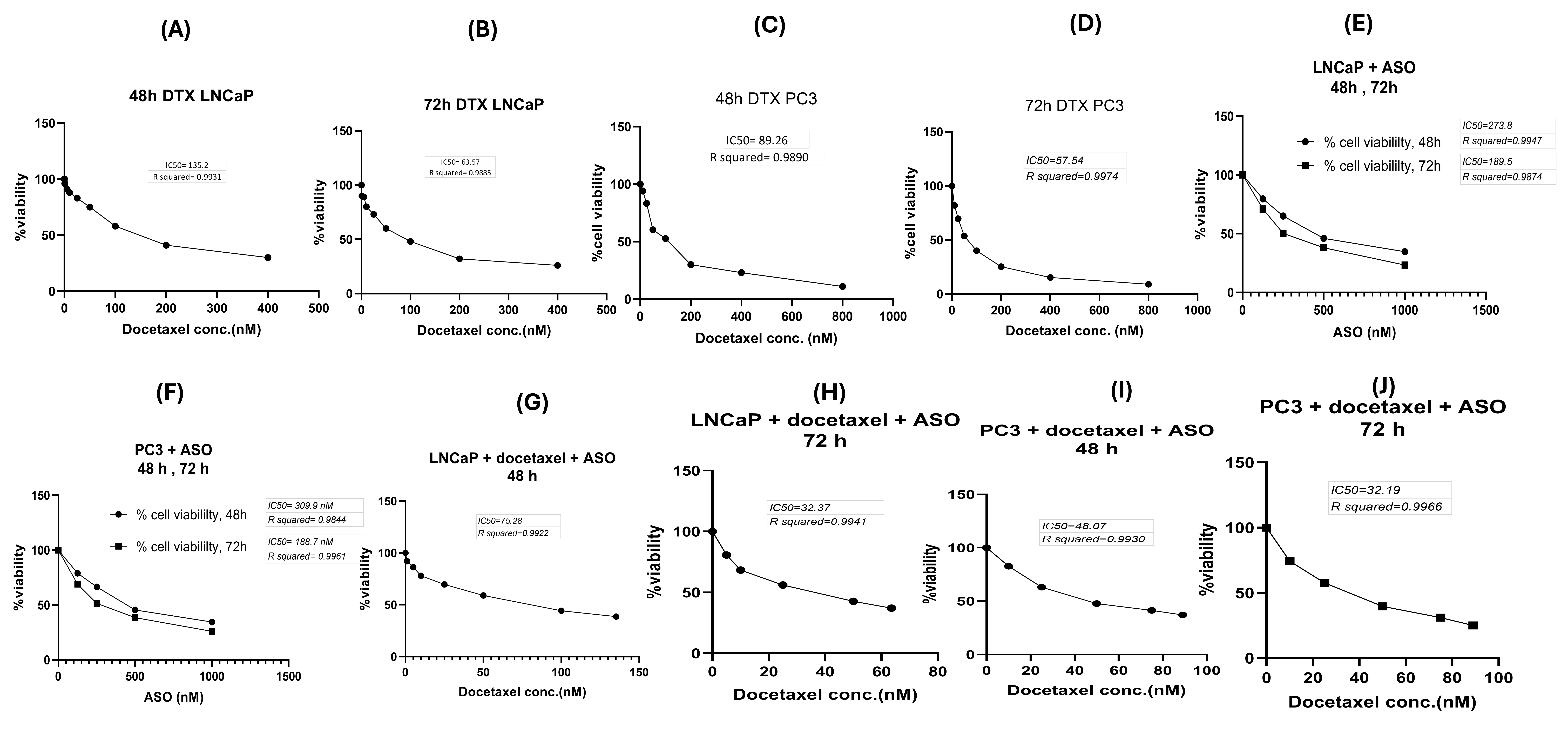
The cytotoxicity of only docetaxel, only ASO and combination of DTX and ASO in PC3 and LNCaP cells line according to the
Apoptosis
In LNCaP cells after 48 h, cell viability was 93 % in the control, 76 % with DTX alone, 74 % with ASO alone, and 16 % with the combination (Figure 7 A–D). In PC3 cells, viability was 91 % in the control, 49 % with DTX, 34 % with ASO, and 13 % with the combination (Figure 7 E–H). The combination exhibited a synergistic effect: the observed fractional inhibition (75 %) exceeded the Bliss-independence value (39 %) (
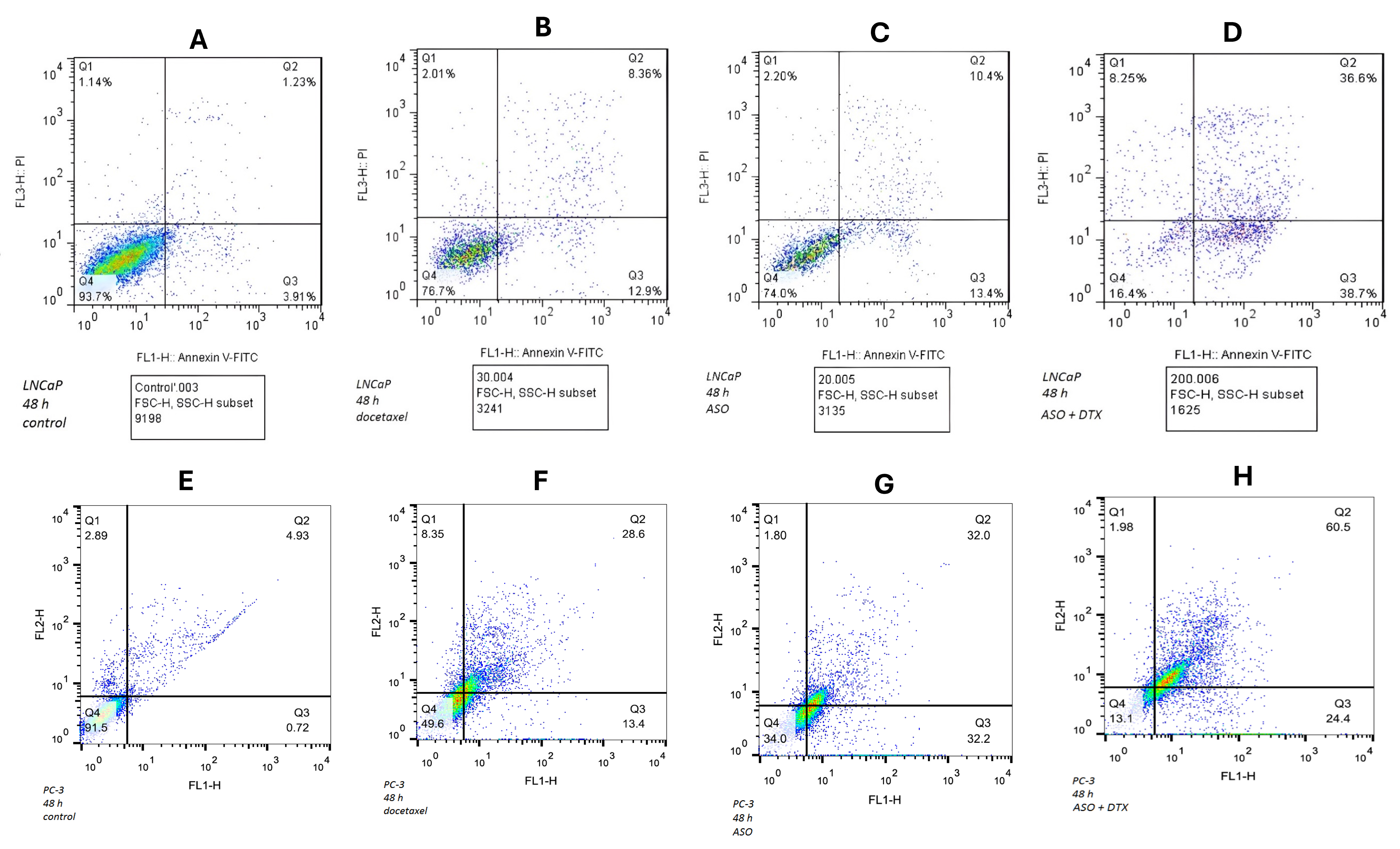
The results of Flow cytometry of LNCaP cell lines. A: The control cells, B: Treated with Docetaxel (DTX), C: Treated with the new designed ASO, D: Treated with the new designed ASO and Docetaxel (DTX). The results of Flow cytometry of PC3 cell lines. E: The control cells, F: Treated with Docetaxel (DTX), G: Treated with the new designed ASO, H: Treated with the new designed ASO and Docetaxel (DTX). Cells were analyzed in independent triplicates of each combination. FL1 channel was used to detect Annexin V-FITC and FL3 indicated PI signals. The viable cells (Annexin V–/PI–) are in the Q4. Early apoptotic cells (Annexin V+/PI+) are in the Q3. Late apoptotic cells (Annexin V+/PI+) are in the Q2 and necrotic cells (Annexin V–/PI+) are in the Q1.
Result of calculating synergistic effect of combination two drugs in LNCaP cell line and in PC3 cell line using bliss independence model
|
Cell line |
ASO (%) |
DTX (%) |
Observed combination (%) |
Bliss expected (%) |
Effect |
|
LNCaP |
23.80 |
21.26 |
75.30 |
40.00 |
Synergy |
|
22.25 |
20.10 |
72.50 |
37.88 |
Synergy | |
|
25.20 |
22.39 |
78.20 |
41.95 |
Synergy | |
|
Mean ASO |
Mean DTX |
Mean Observed combination | |||
|
23.75 |
21.25 |
75.33 | |||
|
SD ASO |
SD DTX |
SD Observed combination | |||
|
1.48 |
1.15 |
2.85 | |||
|
PC3 |
ASO (%) |
DTX (%) |
Observed combination (%) |
Bliss expected (%) |
Effect |
|
64.20 |
42.00 |
84.90 |
79.24 |
Synergy | |
|
61.45 |
40.25 |
81.50 |
76.97 |
Additive | |
|
66.95 |
43.75 |
88.30 |
81.41 |
Synergy | |
|
Mean ASO |
Mean DTX |
Mean Observed combination | |||
|
64.20 |
42.00 |
84.90 | |||
|
SD ASO |
SD DTX |
SD Observed combination | |||
|
2.75 |
1.75 |
3.4 |
Discussion
Prostate cancer is the second most common cancer in men. There are many risk factors for prostate cancer, including age, ethnicity, genetics and family history. Studies show that genetic abnormalities affect not only the androgen receptor but also the regulation of apoptosis1, 2, 3, 4, 5. Prostate cancer can increase the expression of anti-apoptotic genes; it can be immortal as long as the patient lives28. Peer-reviewed studies have reported that defects in apoptotic pathways can cause cancer-cell survival and resistance to chemotherapeutic agents29. BCL-2 plays an essential role in apoptosis30. Overexpression of this gene is associated with resistance to several anticancer therapies31.
It seems that apoptosis induction by chemotherapeutic agents is more effective when combined with BCL-2 silencing. BCL-2 silencing can occur at the mRNA level. Following reduction of BCL-2 expression, cells become vulnerable to therapeutic agents. One method to down-regulate gene expression is antisense or gene-therapy technology. In this approach, single-stranded oligonucleotides (18-21 mer) are designed to hybridize specifically to their region in the mRNA. Next, an RNase H complex cleaves this hybrid. A critical issue in oligo-antisense-based therapy is maintaining oligonucleotide stability in cells at the target site. To address this, researchers have introduced chemical modifications to the oligonucleotide backbone, such as replacement of a phosphate bond with phosphorothioate, base modifications, sulfur substitutions, terminal modifications and 2′-O-methyl changes in the ribose sugar. Another important problem in antisense oligonucleotide therapy is delivery of the oligonucleotide to the target site32, 33, 34, 35, 36.
In this study, to improve stability at the target site, we designed an antisense oligonucleotide containing phosphorothioate at both termini. To address the delivery issue, we used Lipofectamine to carry the newly designed ASO into cells, optimizing cellular uptake and endosomal escape to facilitate membrane crossing. We then investigated the effects of docetaxel and this new antisense oligonucleotide on apoptosis in LNCaP and PC-3 prostate-tumour cell lines. We designed an experiment to evaluate the efficiency of the new ASO in sensitizing cells and inducing apoptosis by down-regulating BCL-2 expression. Two prostate cancer cell lines, LNCaP and PC-3, were used. Four groups of cells were prepared for each line. The first group served as control. Docetaxel was added to the second group. The new ASO was added to the third group. The combination of ASO and docetaxel was added to the fourth group. Real-time PCR was used to assess BCL-2 expression relative to the housekeeping gene GAPDH. Four ASO concentrations were tested for 48 h and 72 h. We selected the 48 h results because after 72 h flow-cytometry analysis showed necrosis in both lines. BCL-2 expression relative to GAPDH in control cells was set to 100 %. After 48 h, BCL-2 expression decreased by ~50 % compared with the control group in both LNCaP and PC-3 lines at 250 nM. Flow cytometry was used to evaluate apoptosis under three conditions: docetaxel alone, ASO alone and the combination, all compared with control. After 48 h, 93 % of control LNCaP cells and 91 % of control PC-3 cells were alive. In groups treated with docetaxel alone, 76 % of LNCaP cells and 49 % of PC-3 cells were alive. In groups transfected with the ASO alone, 74 % of LNCaP cells and 34 % of PC-3 cells were alive. Ultimately, in the combination group, 16 % of LNCaP cells and about 13 % of PC-3 cells were alive33.
Importantly, ASO off-target effects require careful consideration. A systematic approach is crucial, starting with comprehensive in-silico analysis. This should include advanced BLAST searches considering near-cognate sequences, mismatches and wobble base-pairing to predict all potential unintended binding sites across the transcriptome. Empirical validation is then necessary. Statistical rigour is essential to avoid false positives. ASO design must be optimized to minimize off-target effects while maintaining efficacy. Strategies include introducing mismatches at unintended binding sites, using chemically modified ASOs for improved specificity or employing delivery methods that enhance tissue targeting and reduce systemic exposure. An iterative design–test–refine process is crucial for developing a specific and safe ASO.
This study also investigates a synergistic drug-combination strategy to enhance therapeutic effectiveness, reduce dosage and side-effects, and improve patient tolerability compared with monotherapy by accelerating cellular drug entry. In both LNCaP and PC-3 cell lines, a synergistic effect was observed. These findings offer potential for clinical trials designed to overcome DTX resistance. We recognise that our findings, while promising in established cell lines and through in-silico modelling, represent only a preliminary step. The absence of in-vivo validation limits our ability to translate these results directly to the clinic. Future studies are essential to confirm these observations in more physiologically relevant contexts. Specifically, in-vivo experiments using animal models that mimic the human disease are needed to assess efficacy and safety. Investigations using primary cells isolated directly from patients would provide a more accurate representation of disease biology and address potential limitations arising from genetic drift and adaptation of long-term cell lines. These efforts will be crucial for validating our hypotheses and paving the way for potential clinical applications.
Relying on the literature, BCL-2 up-regulation has been invariably implicated in docetaxel resistance across both castration-resistant ( PC-3) and androgen-sensitive models (. LNCaP). For instance, PC-3 sub-lines resistant to docetaxel exhibit elevated BCL-2/BCL-xL levels, while in LNCaP derivatives, androgen-deprivation-induced AR activation correlates with increased BCL-2 expression. Our observation that combining ASO with docetaxel in LNCaP cells lowers the IC₅₀ suggests that ASO may down-regulate this AR/BCL-2-mediated resistance axis, providing a rationale for extending this combination even to androgen-sensitive prostate-cancer models.
Conclusions
Generally, it can be concluded that in most steps of investigation and research work, the newly designed ASO can reduce expression of thegene. The newly designed ASO alone was able to reduce BCL-2 expression significantly. Flow cytometry showed that docetaxel (DTX) induced about 30-40 % of cells to enter the early stage of apoptosis, while the newly designed ASO induced approximately 30-60 %. When the combination of the newly designed ASO and docetaxel (DTX) was used, it induced 80-90 % of cells to enter early apoptosis. This makes it reasonable to conclude that by reducing expression of the BCL-2 gene, cancer cells may be more likely to undergo apoptosis sooner. A more definitive conclusion may be obtained with further studies.
However, validation and primary patient-derived cells were not included in the current study. This constraint was primarily due to limited access to clinical samples, ethical considerations, and resource limitations. We used certified human prostate-cancer cell lines LNCaP and PC3 from a reliable biobank; these models are widely accepted for basic studies. We acknowledge this translational limitation and aim to address our findings in future studies.
Although this study confirmed mRNA down-regulation, potential interactions with other BCL-2 family members such as BCL-xL or MCL-1 were not assessed. This will be examined by RNA-seq or qRT-PCR in future work for further validation.
Abbreviations
ASO - Antisense, Oligonucleotide; BCL-2 - B-cell, Lymphoma, 2; cDNA - Complementary, DNA; DMSO - Dimethyl, Sulfoxide; DTX - Docetaxel; FBS - Fetal, Bovine, Serum; FITC - Fluorescein, Isothiocyanate; GAPDH - Glyceraldehyde, 3-Phosphate, Dehydrogenase; G3139 - Oblimersen; IC₅₀ - Half, Maximal, Inhibitory, Concentration; MFE - Minimum, Free, Energy; mRNA - Messenger, RNA; MTT Assay - (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium, Bromide), Assay; PI - Propidium, Iodide; PS - Phosphorothioate; qRT-PCR - Quantitative, Real-Time, Polymerase, Chain, Reaction; RNA - Ribonucleic, Acid; RPMI-1640 - Roswell, Park, Memorial, Institute, 1640, Medium; SEM - Standard, Error, of, the, Mean; 2'-MOE - 2'-O-(2-methoxyethyl).
Acknowledgments
The authors thank NanoAlvand pharmaceutical company (Iran) for providing Docetaxel (DTX).
Author’s contributions
RA wrote the original manuscript provided laboratory work; MSJ analyzed the data, MM designed tables and scientific illustrations; AASM checked the associated database and raw data and supervised; MR, RG and SS provided laboratory assistant; AM revised the final manuscript. All authors read and approved the final manuscript.
Funding
The Vice Chancellor for Research and Technology of Golestan University of Medical Sciences financed the study. Contract number is 193531. Dr. Ali Akbar Saffar Moghadam received the grant.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The ethics code is http://ethics.research.ac.ir/IR.GOUMS.REC.1398.167. This project did not involve human or animal subjects, nor did it collect data from them.
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have used generative AI and/or AI-assisted technologies in the writing process before submission, but only to improve the language and readability of their paper.
Competing interests
The authors declare that they have no competing interests.

