Relationship of transgelin expression with clinicopathological characteristics and disease-free survival in HER2-positive breast cancer
- Department of Pathology, Hanoi Medical University, Vietnam. 1, Ton That Tung Street, Dong Da District, Hanoi, Viet Nam
- Department of Pathology, Phenikaa University, Vietnam. Nguyen Trac Street, Ha Dong District, Hanoi, Viet Nam
- Department of Pathology, National Cancer Hospital, Hanoi, Vietnam. 43, Quan Su Street, Hoan Kiem District, Hanoi, Viet Nam
- Department of Clinical Pathology, Hanoi Medical University, Viet Nam. 1, Ton That Tung Street, Dong Da District, Hanoi, Viet Nam
- Department of Pathology, Thai Nguyen University of Medicine and Pharmacy, Vietnam, 284 Luong Ngoc Quyen Street, Thai Nguyen City, Thai Nguyen Province, Viet Nam
- Department of Pathology, National Lung Hospital, Ha Noi, Viet Nam, 463 Hoang Hoa Tham Street, Ba Dinh District, Hanoi, Viet Nam
Abstract
Background: Cancer-associated fibroblasts (CAFs) are essential for shaping the tumor microenvironment and influencing therapeutic responses in breast cancer. Thus, a better understanding of the expression of transgelin, a key marker of CAFs, may provide prognostic information. However, the prognostic significance of transgelin in human epidermal growth factor receptor 2 (HER2)-positive breast cancer remains unclear. Here, we investigated the relationship of transgelin expression with clinicopathological characteristics and survival outcomes in HER2-positive breast cancer.
Methods: Data were collected retrospectively from 111 HER2-positive breast cancer tissue samples. The density of tumor-infiltrating lymphocytes (TILs) was evaluated by hematoxylin and eosin staining. Immunohistochemical staining of transgelin and CD8 was conducted.
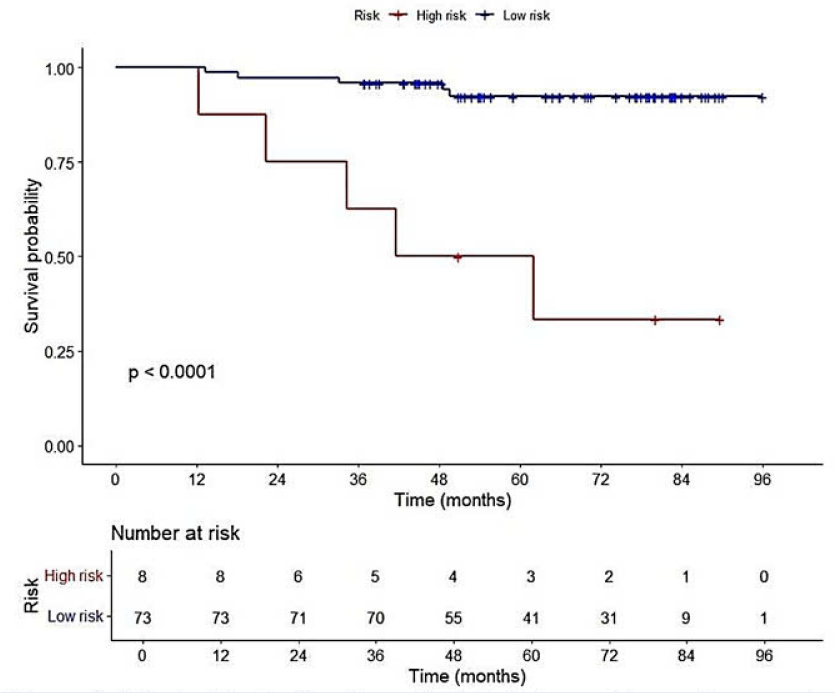
Results: Strong transgelin expression in CAFs was negatively associated with CD8 and TILs (p < 0.005). In the multivariate analysis, transgelin expression in CAFs (p = 0.023) and pathological stage (p = 0.029) were identified as independent prognostic factors for disease-free survival (DFS). The model combining transgelin expression in CAFs and pathological stage improved prediction (−2 log-likelihood = 74.062, Akaike information criterion [AIC] = 78.06) compared with pathological stage alone (−2 log-likelihood = 80.815, AIC = 82.81). Patients in the high-risk group had a shorter DFS (p < 0.0001).
Conclusions: Transgelin expression in CAFs appears to be a novel prognostic marker for HER2-positive breast cancer.
Introduction
HER2-positive breast cancer comprises 25% of all breast-cancer cases and is linked to higher recurrence rates and reduced overall survival (OS)1. Despite trastuzumab therapy, 15–20 % of HER2-positive breast-cancer patients relapse, highlighting the need for better prognostic markers1, 2.
While traditional treatments have primarily targeted tumor cells (TCs), increasing evidence suggests that the tumor microenvironment (TME) plays a crucial role in cancer progression, therapy resistance, and metastasis. The TME comprises various cellular and non-cellular components, including immune cells, stromal cells, extracellular matrix, and cytokines3, 4. Among these, CAFs represent the most abundant stromal cells and have been shown to modulate immune responses and promote resistance to therapy in breast cancer5, 6. Transgelin, a cytoskeletal protein, has been identified as a specific marker for CAFs in several malignancies6, 7, 8.
Due to their important role, CAFs have been the subject of increasing study in recent years5, 6, 7, 8, 9, 10, 11. However, in Vietnam, research on CAFs—particularly their markers (such as transgelin)—remains limited, highlighting the need for further investigation. This study aims to examine the relationship of transgelin expression with clinicopathological characteristics and survival outcomes in HER2-positive breast cancer.
Methods
Collection of clinical samples
In total, 111 mastectomy specimens were collected from HER2-positive breast-cancer patients who had received no pre-operative therapy. Patients with invasive lobular carcinoma, mixed invasive ductal and lobular carcinoma, invasive micropapillary carcinoma, mucinous carcinoma, or inadequate tissue blocks (<5 % tumor/stroma) were excluded12.
Data were abstracted from medical records and included age, tumor size, histology, histologic grade, vascular invasion, lymph-node metastasis, pathological stage, estrogen-receptor (ER) expression, progesterone-receptor (PR) expression, HER2 expression, tumor-cell proliferation index (Ki-67 expression), and treatment regimen.
Unfavorable outcomes comprised ipsilateral locoregional invasive breast-tumor recurrence, contralateral invasive breast cancer, distant disease recurrence, or death from any cause. Overall-survival (OS) and disease-free-survival (DFS) data were obtained from medical records or by patient contact.
All data were collected at the National Cancer Hospital (Hanoi, Vietnam) between 1 January 2017 and 31 December 2021, with follow-up completed by 31 December 2024.
The study was approved by the Hanoi Medical University Ethics Committee (code: IRB-VN01.001/IRB00003121/FWA 00004148) and was conducted in accordance with the Declaration of Helsinki.
Hematoxylin and eosin staining
The hematoxylin-and-eosin (H&E) staining protocol is provided in Supplementary S1.
Histological examination
Whole sections of H&E-stained slides were used to evaluate stromal TILs in strict accordance with the criteria proposed by the International TIL Working Group13. The percentage of all mononuclear cells (including lymphocytes and plasma cells) in the stromal compartment within the border of the invasive tumor was assessed visually. TILs were scored in 5 % increments and classified as <50 % or ≥50 %, following Swisher14.
Immunohistochemical staining
The immunohistochemical (IHC) protocol is provided in Supplementary S2.
CD8 assessments
Whole-section slides were scanned with a Leica Aperio AT2 (40×). CD8 expression on the membrane and in the cytoplasm was analyzed across the entire tumor area using QuPath software15. High CD8 expression was defined as ≥10 % CD8-positive lymphocytes among nucleated stromal cells, as described by L. Zong12.
Transgelin assessments
Transgelin expression in CAFs: nuclear and/or cytoplasmic staining was graded as 0 (no staining), 1 (light brown), 2 (brown), or 3 (dark brown). Grades 0–1 were considered weakly positive, whereas grades 2–3 were considered strongly positive11.
Transgelin expression in TCs: a result was deemed positive when ≥10 % of tumor cells showed dark-brown cytoplasmic staining7. All assessments were performed by two pathologists; discrepancies were resolved by a third pathologist.
Statistical analysis
Data were entered in EpiData 4.6.0.4 (EpiData Association, Denmark) and analyzed in R 4.1.2 (https://www.r-project.org). Associations between transgelin expression and clinicopathological characteristics were examined with the χ² test or Fisher’s exact test. Survival was analyzed with the Kaplan–Meier method and log-rank test. Variables with p < 0.05 in univariate Cox regression and post-hoc power ≥80 % were included in the multivariate model. Model fit was evaluated with −2 log-likelihood and the AIC. Risk groups were compared with the Kaplan–Meier method. A p-value <0.05 was considered statistically significant.
Baseline characteristics of the entire patient cohort (N=111)
|
Parameters |
N |
Percent |
|---|---|---|
|
Age (years) | ||
|
< 50 |
51 |
45.9 |
|
≥ 50 |
60 |
54.1 |
|
Tumor size (cm) | ||
|
< 2 |
32 |
28.8 |
|
2-5 |
79 |
71.2 |
|
Histology | ||
|
Ductal carcinoma |
107 |
96.4 |
|
Others |
4 |
3.6 |
|
Histologic grade | ||
|
1 - 2 |
56 |
50.5 |
|
3 |
55 |
49.5 |
|
Vascular invasion | ||
|
No |
73 |
65.8 |
|
Yes |
38 |
34.2 |
|
Lymph node metastases | ||
|
< 2 |
92 |
82.9 |
|
≥ 2 |
19 |
17.1 |
|
Pathological stage | ||
|
Early stage (IA, IIA, T2N1) |
87 |
78.4 |
|
Locally advanced stage (IIB, T3N0, IIIA-C) |
24 |
21.6 |
|
ER expression | ||
|
Negative |
56 |
50.5 |
|
Positive |
55 |
49.5 |
|
PR expression | ||
|
Negative |
64 |
57.7 |
|
Positive |
47 |
42.3 |
|
HER2 expression | ||
|
IHC 2+ ISH + |
31 |
27.9 |
|
IHC 3+ |
80 |
72.1 |
|
Ki67 expression | ||
|
Low (< 20%) |
7 |
6.3 |
|
High (≥ 20%) |
104 |
93.7 |
|
Transgelin expression in TCs | ||
|
Negative (< 10%) |
90 |
81.1 |
|
Positive (≥ 10%) |
21 |
18.9 |
|
Transgelin expression in CAFs | ||
|
Weak (Grade 0,1) |
74 |
66.7 |
|
Strong (Grade 2,3) |
37 |
33.3 |
|
CD8 expression | ||
|
Low (< 10%) |
80 |
72.1 |
|
High (≥ 10%) |
31 |
27.9 |
|
TILs | ||
|
Low (< 50%) |
98 |
88.3 |
|
High (≥ 50%) |
13 |
11.7 |
|
Trastuzumab | ||
|
No |
59 |
53.2 |
|
Yes |
52 |
46.8 |
Results
Patient characteristics
The distribution of clinicopathological characteristics and transgelin expression is shown in
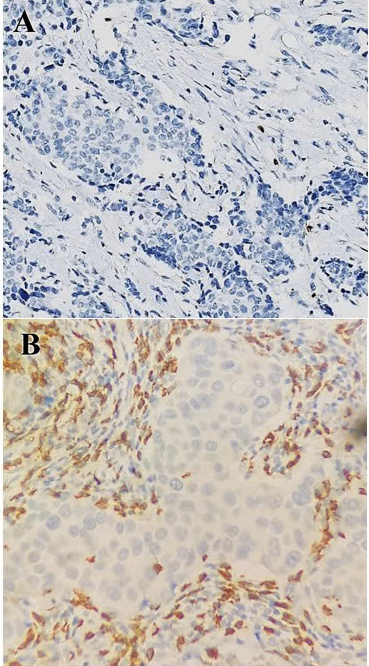
HER2-positive breast cancer. Staining CD8 in original magnification 20x: Low (A), and high (B) expression.
Association of transgelin marker with clinicopathologic characteristics (N=111)
|
Parameters |
Transgelin in TCs positive N (%) |
P-value |
Strong transgelin expression in CAFs N (%) |
P-value |
|---|---|---|---|---|
|
<50 |
9 (17.6) |
0.752 |
17 (33.3) |
1.000 |
|
≥50 |
12 (20.0) |
20 (33.3) | ||
|
Tumor size (cm) | ||||
|
<2 |
5 (15.6) |
0.573 |
8 (25.0) |
0.236 |
|
≥2 |
16 (20.3) |
29 (36.7) | ||
|
Histologic grade | ||||
|
1 - 2 |
7 (12.5) |
0.081 |
20 (35.7) |
0.591 |
|
3 |
14 (25.5) |
17 (30.9) | ||
|
Vascular invasion | ||||
|
No |
11 (15.1) |
0.151 |
31 (33.7) |
0.859 |
|
Yes |
10 (26.3) |
6 (31.6) | ||
|
Lymph node metastases | ||||
|
<2 |
17 (18.5) |
0.755a |
14 (15.2) |
0.507a |
|
≥2 |
4 (21.1) |
4 (21.1) | ||
|
Pathological stage | ||||
|
Early stage (IA, IIA, T2N1) |
13 (14.9) |
0.073a |
28 (32.3) |
0.625 |
|
Locally advanced stage (IIB, T3N0, IIIA-C) |
8 (33.3) |
9 (37.5) | ||
|
ER expression | ||||
|
Negative |
16 (28.6) |
0.009 |
19 (33.9) |
0.893 |
|
Positive |
5 (9.1) |
18 (32.7) | ||
|
PR expression | ||||
|
Negative |
17 (26.6) |
0.016 |
21 (32.8) |
0.892 |
|
Positive |
4 (8.5) |
16 (34.0) | ||
|
Ki67 expression | ||||
|
Low (<20 %) |
0 (0.0) |
0.343a |
3 (42.9) |
0.864a |
|
High (≥20 %) |
21 (20.2) |
34 (32.7) | ||
|
Transgelin expression in TCs | ||||
|
Negative (<10 %) |
29 (32.2) |
0.607 | ||
|
Positive (≥10 %) |
8 (38.1) | |||
|
Transgelin expression in CAFs | ||||
|
Weak (Grade 0,1) |
13 (17.6) |
0.607 | ||
|
Strong (Grade 2,3) |
8 (21.6) | |||
|
CD8 expression | ||||
|
Low (<10%) |
13 (16.3) |
0.249 |
36 (45.0) |
<0.001a |
|
High (≥10%) |
8 (25.8) |
1 (3.2) | ||
|
TILs | ||||
|
Low (<50%) |
18 (18.4) |
0.709a |
37 (37.8) |
0.004a |
|
High (≥50%) |
3 (23.1) |
0 (0.0) |
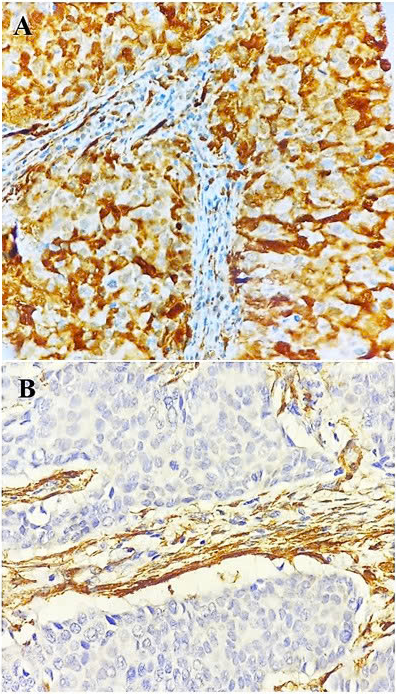
HER2-positive breast cancer. Transgelin expression in tumor cells at original magnification 40x: positive (A); negative (B)
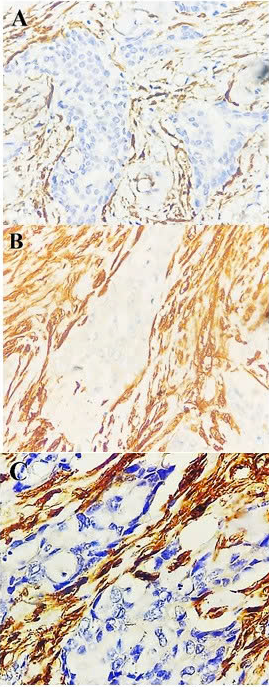
HER2-positive breast cancer. Transgelin expression in cancer-associated fibroblasts at original magnification 40x: Grade 1 (A), grade 2 (B), and grade 3 (C) expression. Negative immunostaining for transgelin was not shown here.
Association of transgelin with clinicopathological characteristics
As shown in
Association of transgelin and clinicopathological characteristics with disease-free survival in HER2-positive breast cancer (N=81)
|
Parameters |
Log rank |
Univariable Cox regression |
Multivariate Cox regression | |||
|
HR (95%CI) |
p-value |
HR (95%CI) |
p-value | |||
|
Age (years) |
< 50 |
0.352 |
1 | |||
|
≥50 |
1.882 (0.486 – 7.283) |
0.360 | ||||
|
Histologic grade |
1 - 2 |
0.708 |
1 | |||
|
3 |
1.247 (0.359 – 4.518) |
0.708 | ||||
|
Vascular invasion |
No |
0.740 |
1 | |||
|
Yes |
1.239 (0.349 - 4.396) |
0.740 | ||||
|
Pathological stage |
Early stage (IA, IIA, T2N1) |
0.032 |
1 |
1 | ||
|
Locally advanced stage (IIB, T3N0, IIIA-C) |
3.561 (1.030 – 12.312) |
0.045 |
4.001 (1.154 – 13.873) |
0.029 | ||
|
ER expression |
Negative |
0.656 |
1 | |||
|
Positive |
1.326 (0.382 – 4.606) |
0.657 | ||||
|
PR expression |
Negative |
0.263 |
1 | |||
|
Positive |
0.423 (0.090 – 1.997) |
0.277 | ||||
|
Ki67 expression |
Low (<20 %) |
0.506 |
1 | |||
|
High (≥20 %) |
0.502 (0.063 – 3.978) |
0.514 | ||||
|
Transgelin expression in TCs |
Negative (<10 %) |
0.543 |
1 | |||
|
Positive (≥10 %) |
0.619 (0.131 – 2.935) |
0.546 | ||||
|
Transgelin expression in CAFs |
Weak (Grade 0,1) |
0.014 |
1 |
1 | ||
|
Strong (Grade 2,3) |
5.559 (1.180 – 26.191) |
0.030 |
6.082 (1.286 – 28.758) |
0.023 | ||
|
CD8 expression |
Low (<10%) |
0.165 |
1 | |||
|
High (≥10%) |
0.258 (0.033 – 2.033) |
0.198 | ||||
|
TILs |
Low (<50%) |
0.288 |
1 | |||
|
High (≥50%) |
0.042 (0.000 – 349.497) |
0.492 | ||||
|
Trastuzumab |
No |
0.955 |
1 | |||
|
Yes |
0.965 (0.278 – 3.348) |
0.955 | ||||
Association of transgelin expression and clinicopathological characteristics with clinical outcomes
The analysis included 81 women with a median follow-up of 65 months (range, 29–96 months). Ten patients (12.3%) experienced recurrence and three (3.7%) died. The 5-year OS and DFS rates were 93% and 84%, respectively. Owing to the low number of deaths, we focused on associations between transgelin expression, clinicopathological characteristics, and DFS. Univariate analysis (
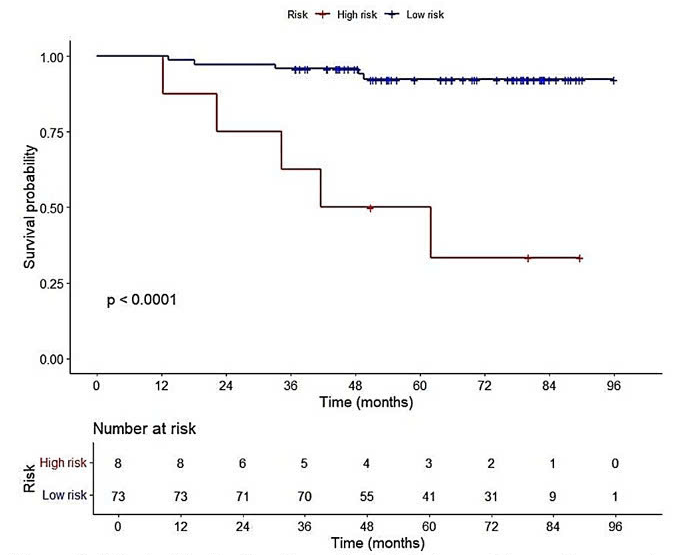
Risk stratification based on pathological stage and transgelin expression in cancer-associated fibroblasts. Kaplan–Meier curves were used to assess survival differences between high and low-risk groups. Patients in the high-risk group had shorter disease-free survival compared to those in the low-risk group.
Additional (Disease-free survival) value of transgelin marker to prognostic multivariable models (N=81)
|
Model variables |
-2 Log Likelihood |
Likelihood ratio P value |
AIC |
|---|---|---|---|
|
Pathological stage |
80.815 |
0.032 |
82.81 |
|
Pathological stage + transgelin expression in CAFs |
74.062 |
0.004 |
78.06 |
Discussion
This study found that strong transgelin expression in CAFs was negatively associated with CD8⁺ cells and TILs. Transgelin expression in TCs was positively associated with ER and PR status. We confirmed that transgelin expression in CAFs and pathological stage were independent prognostic factors for DFS. Including transgelin expression in CAFs in the predictive model provided additional prognostic information beyond pathological stage alone. All patients received no neoadjuvant therapy; stained sections were obtained from resected tumors, accurately reflecting the TME.
In our study, CD8 expression was positively associated with TILs. However, like Liu (2011)16, we did not identify a significant association between CD8 expression and improved DFS. This discrepancy may be attributed to variations in CD8 evaluation methods across studies. Whereas our analysis focused on CD8 in the stromal compartment, other studies have assessed intratumoral compartments or both together17, 18, potentially leading to differing prognostic implications. Furthermore, chronic antigen stimulation within the TME can lead to exhaustion of CD8 effector T cells, reducing their antitumor activity19.
Several studies have investigated the relationship between CAFs and transgelin, a cytoskeletal protein that plays a role in fibroblast activation and tumor progression. Transgelin expression varies between TCs and the surrounding stroma, with significant up-regulation in CAFs compared with TCs. These findings suggest that transgelin is a key marker of fibroblast activation and may contribute to the pro-tumor behavior of CAFs9, 10. Our study showed that transgelin expression was higher in CAFs than in TCs and was significantly associated with short DFS, similar to findings reported previously6, 7, 8
In our study, strong transgelin expression in CAFs was negatively associated with CD8 and TILs, consistent with previous research20, 21. CAFs shape the TME by modulating CD8 responses through antigen presentation and up-regulating immune checkpoints, leading to T-cell impairment and tumor immune evasion22.
Transgelin expression in TCs was positively associated with ER and PR status, in agreement with earlier studies23, 24. However, unlike its prognostic value in CAFs, transgelin expression in TCs was not linked to DFS, suggesting different roles in the stromal and tumor compartments.
Although the number of DFS events was limited, we performed a post-hoc power analysis and model comparisons to enhance the reliability of the findings. Both transgelin expression in CAFs and pathological stage demonstrated sufficient power to detect meaningful associations and were confirmed as independent prognostic factors in the multivariate analysis. Their combination improved model performance, as shown by a lower −2 log-likelihood and the lowest AIC among the tested models. Patients in the high-risk group had a shorter DFS than those in the low-risk group.
This study has several limitations. The small number of DFS events may reduce statistical power and affect the stability of the multivariate analysis. The retrospective, single-center design may also limit generalizability. Finally, the wide confidence intervals (particularly for transgelin) suggest uncertainty in the effect estimates.
Conclusions
Transgelin expression in CAFs may help predict DFS in patients with HER2-positive breast cancer. Further multicenter studies with larger cohorts are needed to validate these results.
Abbreviations
AIC- Akaike information criterion, CAFs- Cancer-associated fibroblasts, CI- Confidence interval, DFS- Disease-free survival, ER- Estrogen receptor, H&E- Hematoxylin and eosin, HER2- Human epidermal growth factor receptor 2, HR- Hazard ratio, IHC- Immunohistochemistry, ISH- In situ hybridization, IQR- Interquartile range, Ki67- Tumor cell proliferation index, OS- Overall survival, PR- Progesterone receptor, TCs- Tumor cells, TILs- Tumor-infiltrating lymphocytes, TME- Tumor microenvironment.
Acknowledgments
The authors thank Nguyen Canh Hiep, Nguyen Thi Khuyen, Nguyen Van Tuan, Nguyen Ngoc Duong, and Mai Thi Nhung for their help during the study.
Author’s contributions
NTT, NVH: Conceptualization, methodology, writing-original draft preparation. NVC, LTT: Visualization, methodology. NTT, NVH, LTT: Data curation, writing-original draft preparation. NVC, LPT: Validation, investigation, supervision, and critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Hanoi Medical University (IRB-VN01.001/IRB00003121/FWA 00004148) and adhered to the Helsinki Declaration.
Consent for publication
Written informed consent was obtained from the patient’s mother for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, .) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

