Investigation of the effect of cerebrospinal fluid from brain-injured rats on the growth and differentiation of neural progenitor cells on neural progenitor cells in vitro
- Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran
- Department of Brain and Cognitive Sciences, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran
- Department of Biology, Roudehen Branch, Islamic Azad University, Roudehen, Iran
Abstract
Introduction: Traumatic brain injury (TBI) encompasses a wide range of brain lesions caused by a blow to or severe impact on the head. Traumatic brain injuries are divided into four categories based on their symptoms and consequences: mild, moderate, severe, and very severe. Studies in animal models have shown that TBI leads to neurodegeneration, progressive brain atrophy, and changes in cerebrospinal fluid (CSF) composition. The aim of this study was to investigate the effect of CSF isolated from TBI victims on neurodegeneration and the differentiation of neural progenitor cells (NPCs).
Methods: To achieve this goal, the expression of genes involved in NPC differentiation and the induction of tauopathy in these cells were examined. First, an animal model of craniocerebral trauma was created in rats, and CSF was collected. The collected CSF was then added to the NPC culture medium to study its effect on cell behavior. The cells were divided into three groups: NPC, NPC + EGF/bFGF, and NPC + EGF/bFGF + CSF. After seven days, the expression of Nestin, Pax6, Map2, and Tubb3 genes was measured by real-time PCR, and TAU expression was evaluated by immunocytochemistry.
Results: A significant decrease in Nestin and Pax6 expression (P = 0.0001 for both) was observed in the experimental group. Map2 and Tubb3 expression increased (P = 0.0001 and P = 0.0002, respectively), indicating NPC differentiation. In addition, immunocytochemistry revealed elevated TAU protein levels (P = 0.05), consistent with tauopathy induction.
Conclusion: We conclude that CSF obtained from TBI rats promotes neuronal differentiation but simultaneously exerts neurotoxic effects through tauopathy induction.
Introduction
TBI encompasses a broad spectrum of brain injuries resulting from a blow or significant impact to the head12. TBI is typically classified into four categories based on clinical presentation: mild, moderate, severe, and very severe34. Common causes include traffic accidents, combat injuries, sports trauma, falls from great heights, and domestic violence. Prompt diagnosis and intervention can reduce TBI severity and sequelae56. According to the World Health Organization, TBI is the third-leading cause of death worldwide. Each year, approximately 1.7 million people sustain a TBI in the United States, whereas >700,000 Iranians are injured in traffic accidents78910. A large proportion of these victims sustain fatal head injuries, with most deaths occurring in the 15–29-year age group11. Because the cellular and molecular mechanisms driving TBI progression remain poorly defined, advances in diagnosis and therapy depend on elucidating these pathways. Animal models enable mechanistic studies and facilitate grading of injury severity. Animal models that develop neurodegeneration—including Alzheimer-like pathology—and progressive atrophy show concomitant alterations in cerebrospinal fluid (CSF) composition121314.
Cerebrospinal fluid (CSF) is a clear, colorless liquid that acts as a shock absorber for the brain and spinal cord15. Research has shown that craniocerebral trauma triggers the migration of neural progenitor cells (NPCs). NPCs are multipotent cells capable of differentiating into both glial and neuronal lineages. Neural stem cells (NSCs) are defined by their abilities to self-renew and to generate multiple daughter cell types. During development, NSCs are responsible for the formation of the central nervous system (CNS)1617. Initially, NSCs give rise to radial glial cells, which themselves serve as an NSC reservoir. Key genes regulating NSC development and differentiation include Nestin (NES), TUBB3, TAU, MAP2, and PAX61819.
PAX6 encodes a transcription factor essential for the development of several organs, notably the brain, eye, olfactory epithelium, and pancreas. It orchestrates neuronal differentiation, migration, and specification20. PAX6 may also maintain neuronal viability, guide circuit formation, and promote astrocyte maturation. Radial glial cells (RGCs) constitute a major NPC population; in the dorsal cortex they express PAX6 but lack intermediate progenitor markers such as Tbr221. PAX6 critically regulates both exit from and re-entry into the cell cycle in cortical progenitors. Loss of PAX6 causes excessive, premature cell-cycle exit22. Chimeric embryos containing wild-type and Pax6 cells confirm that PAX6-deficient progenitors withdraw from the cycle at abnormally high rates early in corticogenesis. Thus, PAX6 is intrinsically required to maintain the cortical progenitor pool23.
TAU stabilizes microtubules, regulates synaptic activity, mediates axonal transport, and modulates MAP-kinase signaling24. Dephosphorylation increases TAU’s affinity for microtubules, thereby enhancing stabilization. In numerous neurological conditions—most notably Alzheimer’s disease—TAU exists in a hyperphosphorylated form, a state referred to as tauopathy. TBI is a major risk factor for Alzheimer’s disease, and abundant evidence associates it with tauopathies25. Animal studies show that TBI induces aberrant phosphorylated TAU accumulation, which triggers neurodegeneration and progressive brain atrophy. Although TBI pathology is multifactorial, tau-mediated mechanisms are considered central to the ensuing neurodegeneration. In humans, TAU is expressed mainly in neurons and astrocytes, whereas in rodents it is also present in oligodendrocytes. Within neurons, TAU is enriched in axons but is also detectable in dendrites, where it supports synaptic function. Post-translational modifications, such as phosphorylation and acetylation, further regulate TAU localization26.
TUBB3 encodes class III β-tubulin, a microtubule component predominantly expressed in neurons. βIII-tubulin participates in neurogenesis, axon guidance, and cytoskeletal maintenance. Its expression peaks during early embryonic neurodevelopment and is retained in induced pluripotent stem cells. High TUBB3 expression persists in both central and peripheral nervous systems27. As an early neural marker, βIII-tubulin is detected at or even before progenitor mitosis. In mice, Tubb3 expression surges during axonal outgrowth, declines in the mature CNS, yet remains high in peripheral neurons. These dynamics suggest tissue-specific regulation. Several transcription factors that govern Tubb3 during neurogenesis have been identified in rat CNS and PNS28.
Neuroepithelial stem cell protein (NES), or nestin, is an intermediate-filament protein. Initially discovered in NSCs, nestin is now known to be expressed in diverse progenitor populations and in tissues including heart, pancreatic islets, and bone marrow. It supports NPC survival, self-renewal, differentiation, and migration and is essential for normal brain and eye development. Within the developing cerebral cortex, horizontal cell divisions are typically asymmetric, producing one migrating neuron and one apical daughter cell that remains in the proliferative zone; in contrast, vertical divisions tend to be symmetric and yield two progenitor cells29. Nestin’s broad expression followed by down-regulation during differentiation suggests a role in lineage specification. Accumulating evidence also implicates nestin in the elevated motility and metastasis of several cancers30.
In vitro studies demonstrate that microtubule-associated proteins (MAPs) bind microtubules and promote their assembly. Among them, TAU is enriched in axons, whereas MAP2 localizes mainly to dendrites. Both proteins attach to tubulin via microtubule-binding domains (MTBDs) containing three or four imperfect repeats. By stabilizing protofilament interactions, MAPs lower the critical tubulin concentration required for polymerization. In CNS neurons, MAP2 is a highly phosphorylated protein present in soma and dendrites. MAP2 exists as three major isoforms—MAP-2a, MAP-2b, and MAP-2c. During rat brain development, MAP-2b remains relatively constant, MAP-2c declines, and MAP-2a rises during synaptogenesis. These isoforms arise by alternative splicing of two transcripts31.
No definitive therapy for TBI currently exists. Deciphering its etiology and molecular mechanisms may yield novel interventions and mitigate its consequences. In particular, the influence of CSF on TBI pathology remains underexplored. Assessing CSF effects on NPCs in injury models could clarify disease mechanisms and suggest therapies. Here, we examined how TBI alters NPC behavior and the expression of differentiation and microtubule-related genes. A TBI model was generated in 2- to 4-month-old male Wistar rats, after which CSF was harvested. The collected CSF was applied to NPC cultures to assess its effects.
Methods
Model Creation Method and Cerebrospinal Fluid Preparation
Male Wistar rats (2 – 3 months old) were obtained from the Royan Research Center and randomly subjected to traumatic brain injury (TBI). The present study was approved by the Ethics Committee under the code IR.IAU.PS.REC.1400.42. Briefly, the method of inducing TBI is as follows: Rats were 6 – 8 weeks old, weighed ~230 g, and were housed under standard conditions. The rats were anesthetized intraperitoneally with xylazine/ketamine (1:10) under Ethics-Committee supervision. After complete anesthesia was ensured, TBI was induced by dropping a 150-g weight onto the skull. After the TBI models regained consciousness, CSF samples were collected from the brainstem of the TBI rats with a needle, transferred to 1.5 mL tubes, and stored at –70 °C. In the next step, NPCs were collected from newborn rats. Finally, the effect of TBI-derived CSF on NPCs was assessed. The cells (N = 12) were randomly assigned to three groups (n = 4 each): negative control (NPCs + genesis factors), positive control (NPCs – genesis factors), and experimental (NPCs – genesis factors + CSF). In total, 12 rats were subjected to TBI, and 6 sham-operated controls were included. Post-operative analgesia and continuous monitoring were provided to minimize suffering.
Cell Culture and Isolation of NPC
Newborn Wistar rats were used to isolate NPCs. The rats were cared for and maintained in accordance with the approved regulations for working with laboratory animals. After complete anesthesia, the hippocampus was separated from both cerebral hemispheres and, after mechanical dissection, the tissue was digested with collagenase for 30 min at 37 °C. FBS was then added to neutralize the enzyme. The resulting suspension was passed through a 70-µm nylon filter and centrifuged at 3000 rpm for 10 min. The cell pellet was cultured in DMEM/F12 medium containing EGF, bFGF, B-27, growth factors, 1 % Penicillin-Streptomycin (Sigma), and 3 % FBS in an incubator at 37 °C and 5 % CO.
Immunocytochemistry
This step comprises the cultivation, fixation, and staining of the cells. A Tau antibody (Cat. No. 136400, Thermo Fisher) was applied to the samples and then washed to remove excess antibody. Images were subsequently captured and analyzed with a light microscope.
RNA Extraction, cDNA Synthesis, and RT-qPCR
RNA was extracted according to the kit protocol using RiboEX solution, cold chloroform, cold isopropanol, ethanol, and refrigerated centrifugation. The quantity and quality of the extracted RNA were determined by NanoDrop and gel electrophoresis, respectively. cDNA synthesis was performed with oligo-dT primers following the protocol of the Easy cDNA Synthesis Kit from Pars Tous (Cat. No. A101161). GAPDH was used as the reference gene. qRT-PCR was carried out with the SYBR Green PCR Kit from Yekta Tajhizma (Cat. No. YT2551) on a Rotor-Gene 6000 instrument (QIAGEN) using the primers listed in
Statistical Analysis
Statistical evaluations were conducted using one-way ANOVA, followed by Dunnett’s multiple-comparison test to assess group differences. Results with p-values below 0.05 were deemed statistically significant. All analyses were carried out using GraphPad Prism version 6.
|
Gene |
Primers |
Tm (°C) |
PCR Product (bp) |
|
Rattus norvegicus tubulin, beta 3 class III (Tubb3), TUJ1 | |||
|
Forward |
GCAACTATGTGGGGGACTCG |
60.0 |
192 |
|
Reverse |
CAGCACCACTCTGACCGAAGA |
57.1 | |
|
Rattus norvegicus microtubule-associated protein 2 (Map2) | |||
|
Forward |
ACTGGAAGAAGCCTCGAAGATG |
60.0 |
158 |
|
Reverse |
TACTTGTGTCCGGCTGAGGA |
60.5 | |
|
Rattus norvegicus nestin (Nes) | |||
|
Forward |
AGCACTCCCATCCCACCTAT |
60.0 |
220 |
|
Reverse |
GGGTTGTGGCTAAGGAGGTC |
60.0 | |
|
Rattus norvegicus paired box 6 (Pax6) | |||
|
Forward |
CCGAATTCTGCAGGTGTCCA |
60.1 |
100 |
|
Reverse |
TGGCTTACTGCCTCCGATTG |
60.3 | |
|
Rattus norvegicus glyceraldehyde-3-phosphate dehydrogenase (Gapdh) | |||
|
Forward |
GAAGCTGGTCATCAACGGGA |
60.0 |
180 |
|
Reverse |
GAAGGGGCGGAGATGATGAC |
60.2 | |
Results
MTT Results
The toxicity of cerebrospinal fluid (CSF) on neural progenitor cells (NPCs) was assessed using the MTT assay. Five plates were prepared, each receiving a different CSF concentration, and viability was measured after 24, 48, and 72 h. The control plate received no CSF and showed 100 % viability. The experimental plates received CSF at final concentrations of 15 %, 25 %, 50 %, and 75 %. Viability remained close to 100 % in every group. Consistent with previous studies, 50 % CSF was selected for subsequent experiments (Figure 1). Nevertheless, the unexpectedly high viability—even at 75 % CSF for 72 h—indicates a possible technical error, which we acknowledge as a limitation.
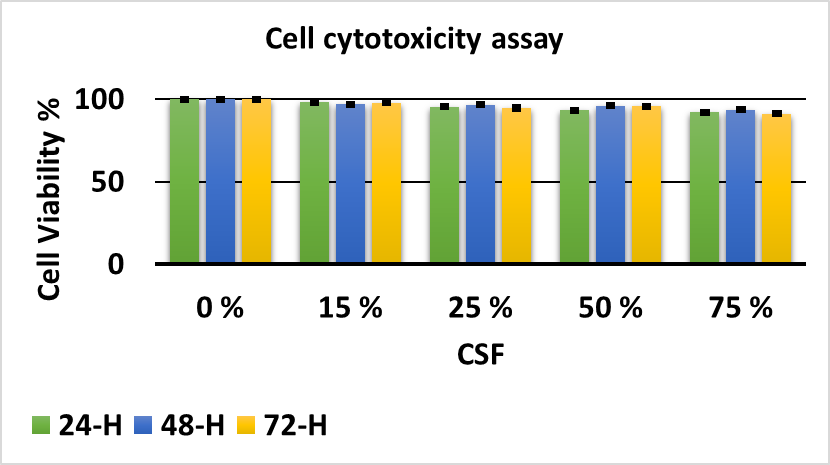
Study of the effect of CSF toxicity on NPCs
Immunocytochemistry
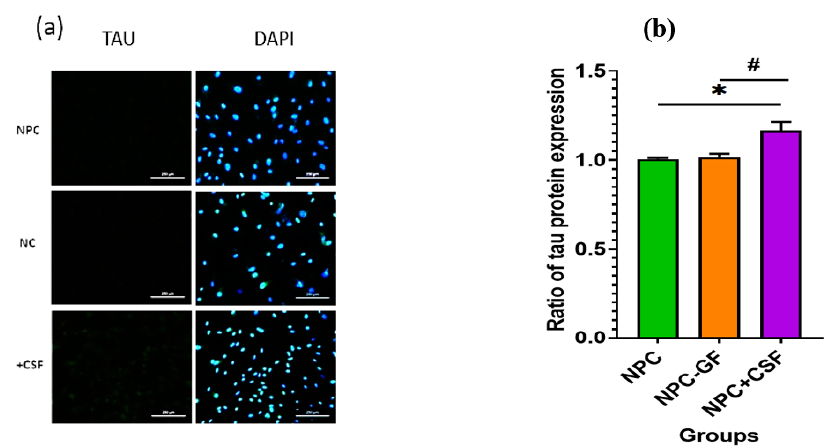
Immunocytochemistry was performed to evaluate TAU protein expression in two control groups and one experimental group. As shown in Figure 2a, TAU expression increased (P = 0.05) in samples exposed to injury-derived CSF for one week. Figure 2b presents ImageJ-based quantification of TAU expression.
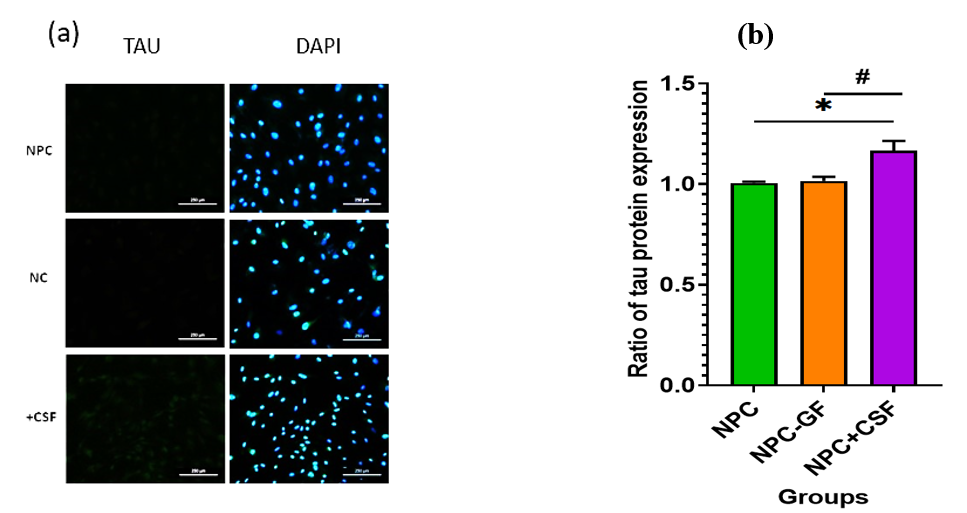
Immunocytochemistry was performed to evaluate TAU protein expression in two control groups and one experimental group. (a) Immunofluorescence staining after co-culture with TAU antibody. (b) Graphical representation of TAU expression in NPCs of the target group with the addition of (NPC-EGF/bFGF+CSF) compared to the control groups of NPCs without the addition of CSF with and without the addition of EGF and bFGF (NPC-EGF/bFGF, respectively).
Verification of real-time PCR products
After real-time PCR, all amplicons were resolved on a 2 % agarose gel to confirm the absence of primer-dimers and non-specific products (Figure 3).
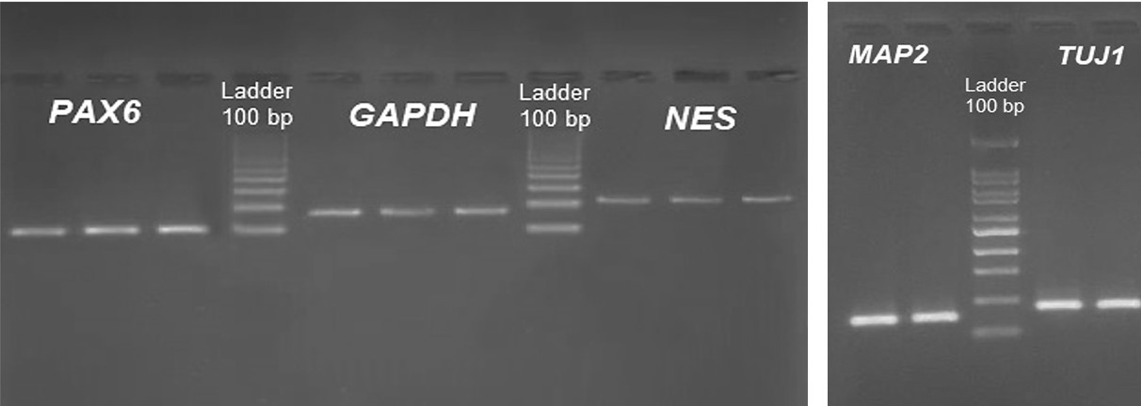
Results of gel-electrophoresis analysis to ensure the absence of primer-dimers and non-specific product in the RT-qPCR reaction.
Relative gene-expression analysis
Because amplification efficiencies were ≈ 2, the 2 (Livak) method was applied. Statistical analyses were performed in SPSS using one-way ANOVA, followed by Tukey’s post-hoc test. As shown in Figure 4, Pax6 and Nestin expression decreased significantly (P = 0.0001) after CSF exposure, whereas Map2 and Tubb3 expression increased significantly (P = 0.0001).
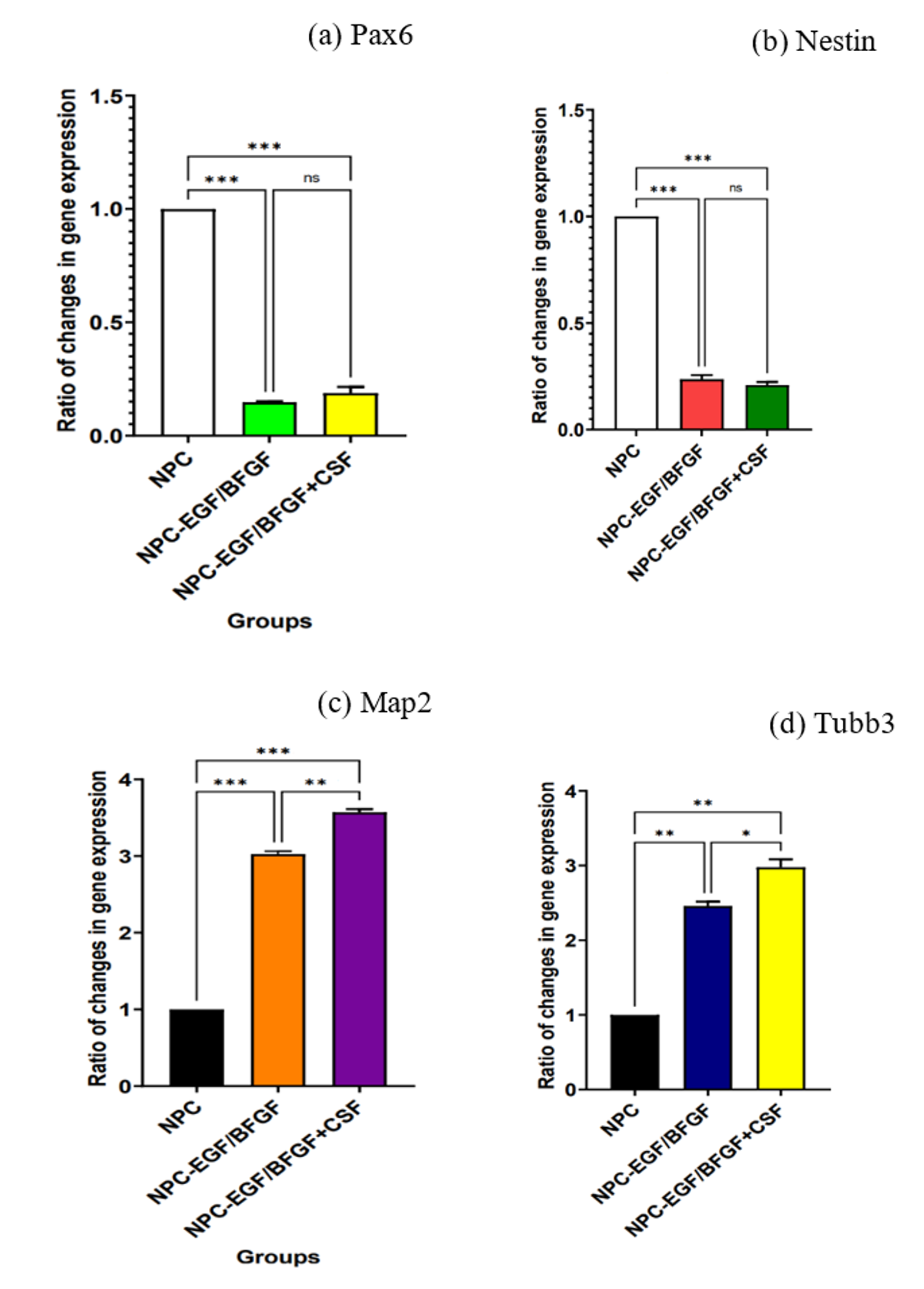
Relative gene-expression analysis. (a) Graph of Pax6 expression in neural progenitor cells of the target group with the addition of (NPC- EGF/bFGF+CSF) compared to the NPC control groups without the addition of CSF with and without the addition of EGF and bFGF (NPC-EGF/bFGF and NPC, respectively). (b) Diagram of Nes expression in neural progenitor cells of the target group with the addition of (NPC- EGF/bFGF+CSF) compared to the control NPC groups without the addition of CSF with and without the addition of EGF and BFGF (NPC-EGF/bFGF and NPC, respectively). (c) Diagram of Map2 expression in neural progenitor cells of the target group with the addition of (NPC- EGF/bFGF+CSF) and the NPC control groups without the addition of bFGF and EGF (NPC and NPC-EGE/bFGF, respectively). (d) Diagram of Tubb3 expression in NPCs of the target group with the addition of (NPC- EGF/bFGF+CSF). Compared to the NPC control groups without the addition of CSF with and without the addition of BFGF and EGF (NPC and NPC-EGF/bFGF respectively).
Discussion
Traumatic brain injury (TBI) is a significant public health concern and one of the leading causes of mortality and disability worldwide. It results in both immediate and long-term neurological deficits. Each year, more than 50 million people worldwide sustain a TBI32. Blast exposures and concussive impacts are among the leading causes of TBI, producing varying degrees of cerebrovascular injury, white- and gray-matter damage, and neuronal loss. TBI is also associated with post-traumatic stress disorder, memory impairment, chronic traumatic encephalopathy (CTE), and persistent neuroinflammation33.
Numerous studies have reported an increased risk of neurodegenerative diseases after TBI. Acute axonal and neurotransmitter damage following TBI constitutes an environmental risk factor for Alzheimer's disease, whose hallmark is tauopathy resulting from hyperphosphorylated tau. In vitro, neurons from rat models of TBI rapidly produce an isomer of phosphorylated tau called cis p-Tau, which disrupts axonal microtubules and leads to apoptosis, a process termed cis-Tauosis. In general, many metabolic changes occur after TBI34, involving amino-acid, carbohydrate, and lipid metabolism and potentially affecting the brain and other organs. Collectively, these changes contribute to clinical manifestations ranging from motor impairments to severe neurocognitive and personality disorders. Cerebrospinal fluid (CSF) acts as a signaling medium within the brain; therefore, leveraging CSF to restore central-nervous-system homeostasis may provide novel therapeutic opportunities for TBI and other neurodegenerative diseases.
TBI can alter CSF composition—for example, by elevating specific proteins and inflammatory mediators. These changes can serve as biomarkers for the diagnosis and monitoring of TBI. CSF factors can also detect transient changes in mild TBI (mTBI) and chronic post-TBI states. Neurogenesis may be accompanied by immune responses that modify CSF inflammatory profiles. Abnormal protein accumulation in Alzheimer's and Parkinson's disease likewise affects CSF composition, prompting investigation of CSF-directed therapies35. Agents that modulate inflammation or promote neuronal regeneration could improve outcomes after TBI. Accordingly, CSF analysis provides valuable information for diagnosis, prognosis, and therapeutic monitoring. However, redundancy in current literature should be minimized; “CSF factor testing” already encompasses these applications.
To examine how TBI-derived CSF influences neurogenesis and neuronal loss, we exposed neural progenitor cells (NPCs) to CSF collected from injured rats. Our primary objective was to determine whether post-TBI CSF impairs differentiation and induces tauopathy—effects that could extend to pregnant females with TBI. Our results indicate that TBI induces CSF alterations that drive NPCs out of the progenitor state, promote differentiation, and damage mature neurons, leading to increased tau. Genes governing differentiation were up-regulated in the experimental group, suggesting that some progenitors continued along the neuronal lineage despite the hostile milieu.
As expected for loss of stemness, the precursor markers Pax6 and Nestin were down-regulated, whereas the neuronal markers Map2 and Tubb3 were up-regulated. Pax6 regulates neuron maintenance, proliferation, and differentiation in the adult brain36 and is highly expressed in NPCs but reduced in mature tissue37. In 2017, Salmin et al. reported decreased Pax6 expression in a mouse model of Alzheimer's disease. Consistent with those data, we observed diminished Pax6 expression in CSF from rats with TBI.
Nestin is expressed in neural stem/progenitor cells and supports proliferation and differentiation. Its CSF level varies with age, sex, and injury severity. Kaya et al. reported low basal Nestin expression that increased after TBI. Kaneko’s group observed a transient Nestin peak 8 h after trauma that returned to baseline thereafter38. In 2018, Yu et al. found reduced Nestin expression in an Alzheimer’s model39.
In our model, TBI-CSF added to the culture medium down-regulated Pax6 and Nestin, mirroring progenitor loss, while withdrawal of growth factors produced a similar effect. Conversely, Map2 and Tubb3 were up-regulated, confirming neuronal differentiation. Map2 is essential for cytoskeletal stability; disruption of this network contributes to neurodegenerative disease. Both Map2 and Tubb3 are widely used neuronal markers.
Papa . showed that CSF Map2 peaks 72 h after severe brain injury and declines within 10 days40. Goodson . (2018) found reduced Map2 expression after TBI in adult male mice, and similar reductions were observed in Alzheimer’s patients41.
Chronic traumatic encephalopathy (CTE) is associated with repeated head trauma and shows reduced Tubb3 expression, whereas Zhenghui et al. reported elevated Tubb3 after single-event TBI42. In our study, TBI-CSF increased Map2 and Tubb3 expression in NPC cultures, indicating that CSF can promote neuronal differentiation despite its cytotoxic components.
Tau expression was used to detect tauopathy. Tau stabilizes axonal microtubules, and its hyperphosphorylation is characteristic of several neurodegenerative diseases. Changes in Tau concentration or phosphorylation in CSF are valuable biomarkers. Shekhar et al. reported elevated CSF Tau in Alzheimer’s patients43.
TBI is a risk factor for Alzheimer’s disease and CTE. In young rats, Tau expression rose one day after TBI and peaked a few days later. In our cultures, TBI-CSF increased Tau expression and induced tauopathy. Previous studies likewise demonstrated elevated CSF Tau after brain injury and in multiple sclerosis, where it correlates with disability.
Neurofilament (NF) proteins released into CSF reflect axonal damage and neuronal death; thus, any disorder causing axonal injury can raise CSF NF levels. CSF participates in neurogenesis by delivering growth factors and signaling molecules to neural stem cells, and compositional changes after TBI can shift this balance. In line with our findings, TBI-CSF decreased the progenitor markers Nestin and Pax6 while increasing the differentiation markers Map2 and Tubb3 and the pathological marker Tau, thereby linking CSF alterations to both neurogenesis and tauopathy.
Conclusions
During craniocerebral trauma, the brain undergoes alterations that change the properties, content, and composition of cerebrospinal fluid (CSF). These modifications promote neural progenitor cell differentiation and concurrently trigger tauopathy. Our results indicate that CSF from traumatic brain injury (TBI) patients diminishes the brain’s regenerative capacity and contributes to the chronic neurological disorders observed after TBI. Future studies should elucidate the mechanisms by which CSF drives neural progenitor cell (NPC) changes and develop therapeutic strategies to prevent or reverse these effects.
Abbreviations
B-27: B-27 Supplement; bFGF: basic Fibroblast Growth Factor; CNS: Central Nervous System; CSF: Cerebrospinal Fluid; CTE: Chronic Traumatic Encephalopathy; DMEM/F12: Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12; EGF: Epidermal Growth Factor; FBS: Fetal Bovine Serum; GAPDH: Glyceraldehyde-3-Phosphate Dehydrogenase; MAP2: Microtubule-Associated Protein 2; MAPs: Microtubule-Associated Proteins; MTBDs: Microtubule-Binding Domains; MTT: (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide); mTBI: mild Traumatic Brain Injury; NF: Neurofilament; NPCs: Neural Progenitor Cells; NSCs: Neural Stem Cells; Pax6: Paired Box 6; PCR: Polymerase Chain Reaction; PNS: Peripheral Nervous System; RGCs: Radial Glial Cells; RT-qPCR: Real-Time Quantitative Polymerase Chain Reaction; TAU: Tubulin-Associated Unit; TBI: Traumatic Brain Injury; Tubb3: Tubulin Beta 3 Class III
Acknowledgments
We would like to thank Royan Institute stuff for their assistance to this project.
Author’s contributions
R.S. performing the main steps of essay, writing the manuscript draft, collecting the samples. E.E. and K.S. RNA extraction, ICC, qPCR and statistical tests. A.E. Head of team and monitoring all steps of the experiment. The authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee under the code IR.IAU.PS.REC.1400.42. In total, 12 rats were subjected to TBI and 6 sham-operated controls were included. Post-operative analgesia and continuous monitoring were provided to minimize suffering.
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc.) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

