An assessment of the performance and effectiveness of collagenase types in quality improvement of small extracellular vesicles from human adipose-derived stromal vascular fraction for precision medicine applications
- Department of Microbiology, Central Research Laboratory and Nitte University Centre for Stem Cell Research & Regenerative Medicine (NUCSReM), KS Hegde Medical Academy (KSHEMA), Nitte (Deemed to be University), Deralakatte, Mangaluru 575018, India
- Department of Plastic Surgery, KS Hegde Medical Academy (KSHEMA), Nitte(Deemed to be University), Deralakatte, Mangaluru 575018, India
- Nitte University Centre for Stem Cell Research & Regenerative Medicine (NUCSReM), KS Hegde Medical Academy (KSHEMA), Nitte (Deemed to be University), Deralakatte, Mangaluru 575018, India
- Central Research Laboratory, KS Hegde Medical Academy (KSHEMA), Nitte (Deemed to be University), Deralakatte, Mangaluru 575018, India
- Centre for Molecular Neurosciences, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, 576104, India
Abstract
Introduction: Small extracellular vesicles (sEVs) are gaining recognition as promising tools for medical applications due to their natural compatibility with biological systems and potential for targeted delivery. Among the various sources, tissue-derived sEVs (ti-sEVs) offer unique properties compared to vesicles from other origins. However, the dense extracellular matrix (ECM) within tissues may trap sEVs, limiting their yield and purity. These limitations remain major obstacles to clinical translation, as therapeutic efficacy depends on abundant, high-quality vesicles capable of efficient cargo transfer. Efficient isolation strategies are therefore critical.
Methods: In this study, we focused on the stromal vascular fraction (SVF) of healthy human adipose tissue (hHAT), a heterogeneous cell mixture that retains properties of its tissue of origin. SVF is typically isolated by enzymatic digestion; yet, the impact of different collagenases on sEV quality and yield is unclear. To address this, we examined the effects of collagenase type II and type IV on SVF cells and their release of sEVs in vitro. SVF-sEVs were quantified after 16–24 h of conditioning and characterized by standard assays—including cell-viability assays, nanoparticle tracking analysis, transmission electron microscopy, fluorescence microscopy, and western blotting—following MISEV guidelines.
Result: Across six hHAT-SVF samples, collagenase type II produced a higher yield (6.94 particles/ml vs. 4.55 particles/ml with type IV) while preserving vesicle integrity and reducing contamination.
Conclusion: These findings indicate that collagenase type II is a gentler and more effective option, significantly enhancing SVF-sEV yield and quality.
Introduction
Extracellular vesicles (EVs) are diverse, membrane-bound entities released by active cells into their surroundings. These vesicles contain a wide range of microRNAs, messenger RNAs, proteins and bioactive lipids that reflect the characteristics of their parent cells and thereby mediate various biological functions1. Among the different types of EVs, small extracellular vesicles (sEVs)—typically 50–200 nm in diameter—have gained significant attention owing to their stability and potential therapeutic applications. Their remarkable adaptability and exceptional biocompatibility make them stand out as cell-free natural nanoparticles, offering exciting opportunities for innovative solutions in biomedicine, particularly in the fight against antibiotic-resistant microorganisms (ARM)2345. Within this area of research, tissue-derived small extracellular vesicles (ti-sEVs) are particularly valuable because they provide insights into tissue microenvironments and disease progression and offer advantages over sEVs sourced from cell-culture supernatants or bodily fluids. However, their potential remains largely untapped because extracting them from dense extracellular matrices (ECM) is challenging; these matrices trap vesicles and impede their release. In addition, the successful clinical application of sEVs hinges on obtaining vesicles with adequate therapeutic cargo; therefore, constraints on yield and vesicle loss during tissue processing are significant hurdles6789. Consequently, developing strategies for the large-scale production of highly bioactive sEVs is crucial. Although several protocols for ti-sEV isolation have been described, each carries inherent limitations: commercial kits are expensive, whereas mechanical methods can damage sEVs, increase contamination risk and yield inconsistent purity10. These challenges underscore the urgent need for an optimized, cost-effective isolation strategy that promotes the release of large quantities of sEVs from tissue cells11.
In this context, healthy human adipose tissue (hHAT)—specifically its stromal vascular fraction (SVF)—emerges as an ideal source. hHAT is increasingly recognized for therapeutic use because of its immunomodulatory properties and the ease of minimally-invasive procurement. The SVF contains a rich array of stem and progenitor cells that mirror the characteristics of the parent tissue and offer ethical advantages over traditional stem-cell sources12131415.
The sEVs secreted by SVF cells inherit the biological cargo of their parent tissue, making them suitable for targeted therapy. Therefore, efficient extraction and purification of SVF-sEVs are critical to maximizing their medical benefits. However, adipose-tissue ECM is rich in lipid-laden adipocytes, which hampers SVF-sEV enrichment and purification1516.
Moreover, contamination by intracellular vesicles or vesicle-like debris released from ruptured cells must be minimized. Even a small number of dying cells can emit large quantities of particles that mimic genuine EVs, complicating the production of pure SVF and sEV preparations.
Validation studies comparing isolation methods for high-yield, clinical-grade SVF-sEVs are scarce, leaving uncertainty about the most effective strategy. The isolation procedure strongly influences the biological activity of SVF-derived sEVs and, ultimately, their clinical efficacy. Refining the tissue-digestion step is therefore essential for preserving cellular integrity, reducing contamination from lysed cells and improving SVF-sEV purity.
This study evaluates whether isolating SVF-sEVs with a specific collagenase, combined with microenvironment-tailored modifications, improves time efficiency, feasibility, applicability and cost relative to other enzymatic and mechanical approaches. We hypothesize that a higher yield of purer ti-sEVs/SVF-sEVs from hHAT will translate into substantial medical benefits.
Historically, tissue processing has relied on mechanical dissociation, homogenization or enzymes such as dispase, trypsin and collagenase (types I and IV), often compromising vesicle integrity and yield. Reports indicate that collagenases preserve vesicle structure at concentrations of 0.075–0.3 % (w/v)171819 .
To overcome existing limitations, we propose a refined digestion protocol using collagenase types II and IV. By efficiently degrading native collagen in the hHAT ECM, this approach enhances SVF isolation and subsequently the release of SVF-sEVs.
The overarching goal is to explore the immunomodulatory and antimicrobial potential of hHAT-derived SVF-sEVs. Our specific aims are to:
• Develop a robust, reproducible protocol for SVF-sEV isolation;
• Optimize enzyme type, concentration and incubation time, and refine downstream isolation techniques;
• Establish a streamlined, cost-effective, single-step workflow that yields SVF-sEVs of high purity and structural integrity20.
Methods
Ethical considerations
This study was performed in accordance with the principles of the Declaration of Helsinki (2004). Approval was granted by the Central Ethics Committee (Date: 13 Jan 2022 / No. NU/CEC/2021/230).
Type of Sampling
SVF from healthy human adipose tissue (hHAT) / cell medium.
Eligibility criteria
Consenting healthy Indian adults aged 18–45 years with a BMI < 30 kg m⁻² were included. We considered 3600 harvesting sites. Individuals outside this age range, with significant medical history or comorbidities, a BMI > 30 kg m⁻², or unwilling to provide consent were excluded.
Study design
In-vitro, original study.
Sample size: six subjects (X females, Y males). Type II and type IV collagenases (tissue-digestion enzymes, TDEs) were tested in triplicate for their ability to enhance SVF-sEV yield and purity. Differential ultracentrifugation (DUC) was then performed at various speeds and durations, and conditioning-time stratification was evaluated for the SVF medium.
Protocol and data collection
Fresh, filtered phosphate-buffered saline (PBS), Dulbecco’s Modified Eagle Medium (DMEM) without FBS (control), 1 % penicillin/streptomycin (PS) or antibiotic/antimycotic solution, 0.1 % Gibco™ collagenase (Type II, Cat. #17101015; Type IV, Cat. #17104019; single lot), and erythrocyte-lysis solution were used21.
Using an established protocol, 150 mL of hHAT lipoaspirate were collected from each of the six subjects. Samples were processed immediately or within ~12 h at 4 °C after delivery to the cell-processing laboratory. Samples were transferred from glass containers, aliquoted into 50 mL Falcon tubes, and subjected to several rounds of DUC.
Samples were washed at 500 × g for 5 min with an equal volume of freshly prepared 0.22 µm-filtered PBS to remove blood, mucus, and anesthetic solution.
The first three pairs of aliquots received 0.1 % collagenase IV to test enzyme effects and were incubated 4.5 h at 37 °C with moderate shaking (35 rpm). Remaining aliquots were digested with 0.1 % collagenase II under the same conditions. Enzyme activity was stopped with cold PBS; stromal cells were pelleted and interstitial fluid harvested.
SVF isolation, cell counting, and viability analysis
After pelleting, cell counts and viability were determined immediately by trypan-blue exclusion using an automated counter. Before viability assessment, pellets were treated with erythrocyte-lysis solution for 10 min at 37 °C, centrifuged, washed with PBS, and strained through a 100 µm mesh.
Conditioning of SVF, and isolation/enrichment of SVF-sEVs
Approximately 6 × 10⁶ SVF cells were seeded per T75 flask in DMEM (no FBS) plus 1 % PS and cultured at 37 °C, 5 % CO for 24 h or until 50–80 % subconfluence22.
Conditioned medium was collected at 16 h and 24 h, transferred to 50 mL tubes, and processed in two isolation phases:
The first phase included first centrifugation at low speed 1500 x g for 5 min at 37 °C where sEVs were separated from cell components (alive and dead) [e.g., cell debris and cellular organelles were removed] and second centrifugation at high speed 14000 x g for 15 min at 4 °C, in clicklock centrifuge tubes, where remaining cell debris / apoptotic bodies, larger EVs and proteins were pelleted, resulting in a cleared concentrated conditioned medium.
The second phase involved the swinging-bucket-based ultracentrifugation at ultra-high speed 200000 x g for 70 min at 4°C and subsequent discarding of supernatant (Sup) and finally, washing of the pellet with plain PBS by ultracentrifuging again at 100000× g at 4 ◦C for 70 min. Twice DUC process resulted in the production of pure and enriched SVF-sEVs from non-cultivated hHAT, following good manufacturing practices and good clinical practice guidelines23. This involved several attempts to ensure accurate results.
Cryopreservation and thawing
Pellets were resuspended in an equal volume of PBS and stored at −80 °C for long-term use or at 4 °C for immediate analysis24.
Characterization and quantification of SVF-sEVs
Isolates were characterized per MISEV guidelines2526.
Half the initial aliquots were treated with collagenase IV and half with collagenase II; enriched SVF-sEVs (Pellet) were characterized after DUC and ultrafiltration.
Phenotypic quantification
For these isolates, zeta potential (ZP) and nanoparticle tracking analysis (NTA) were performed immediately. Transmission electron microscopy (TEM) and fluorescent microscopy (FM) were conducted within two months of storage, and western blotting (WB) was performed on samples thawed after four months. Subsequently, aliquots of the latter samples were treated with collagenase type II only; characterization tests for these isolates were carried out as soon as possible. It is also worth noting that additional NTA measurements were taken after months of storage (before total protein estimation and protein expression tests) to compare the purity of fresh and older SVF-sEV preparations.
ZP: Colloidal Stability: This analysis determined the surface charge (zeta potential, ZP) of isolated SVF-sEVs at 25°C with specific settings, including a shutter speed of 70, sensitivity of 85, and a frame rate of 30 fps. Data were collected and analyzed using ZetaView software (data not shown). Evaluating the surface charge of nanoscale sEV particles in an SVF colloidal system via ZP is critical for predicting their long-term stability, as lipids, pH, salts, and detergents can alter the composition of SVF-sEVs during isolation, potentially influencing their biological activity. A ZP of -60 to +60 millivolts (mV) is ideal for stability, while a ZP of -10 to +10 mV increases the likelihood of sEV aggregation27.
NTA: Size and Concentration: The average size and concentration of bulk SVF-sEVs were measured using a NanoSight LM10 system with a 405 nm laser (Malvern Instruments, UK). Samples were vortexed and diluted 1:10,000 in sterile DPBS to prevent clumping and ensure homogeneous particle distribution. To achieve a particle concentration of 20-100 particles per frame, SVF-sEV samples were diluted with 0.1 μm-filtered PBS and injected into the sample chamber using a sterile syringe. The instrument parameters were calibrated using NIST-traceable 200 nm polystyrene beads (3000 series; Thermo Scientific, Waltham, MA, USA) dissolved in 10 mM potassium chloride. Each sample was recorded with a detection threshold of 7 and a camera level of 1428.
FM: Morphology and Viability: DAPI dye was obtained from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA). In a clean 1.5 ml tube, 20 μl of freshly isolated SVF-sEVs was gently vortexed with 20 μl of diluted dye stock. The mixture was then vortexed again and incubated for 20 minutes at 37°C in the dark. Next, 10 μl of the mixture was placed on a glass slide, and a coverslip was carefully positioned over it using tweezers. The slide was placed on the fluorescent microscope stage, and after a focal plane was identified, several images were captured to show a distribution of diffracted points of interest across the field of view29.
TEM: Ultrastructure: Following the bulk evaluation by NTA, the SVF-sEVs underwent single-particle measurements and morphological stability evaluation via TEM to confirm their presence, membrane integrity, potential disruption, and diameter profiling. A 100 μl aliquot of the SVF-sEV pellet (Pellet, freshly isolated by differential ultracentrifugation (DUC) and resuspended in PBS) was diluted at a 1:5 ratio with dispersant/nano-water. This dilution was applied to carbon-formvar-coated grids (Ted Pella Inc., Redding, CA, USA) that had undergone a 5–10 minute glow discharge. After blotting and washing, the grids were stained with 0.5% uranyl acetate (Electron Microscopy Sciences, Hatfield, PA, USA) for 10 minutes, washed gently, air-dried, and imaged using an FEI Tecnai T20, 200 keV multifunctional analytical transmission electron microscope. TEM images showed that the SVF-sEV samples consisted primarily of an sEV population, as indicated by their size, shape, contrast, and the presence of a defined border30.
WB: Protein Quantification: The diluted, purified SVF-sEV fractions were concentrated using an Amicon® Ultra-15 centrifugal filter unit (10 kDa cutoff; Millipore, Merck KGaA, Ireland) by centrifugation at 4000 × g for 30 minutes at 4°C. The concentrated sample was transferred to a fresh tube, and sEV proteins were lysed with RIPA buffer containing a protease inhibitor cocktail (Sigma-Aldrich) before sonication. To ensure homogeneous distribution of sEVs and maintain sample quality, sonication was performed using a Labman Scientific Instruments pro650 sonicator with a 3 mm probe at specific settings (20% amplitude, 4 seconds on, 2 seconds off, for 3 cycles)31.
The SVF-sEV samples were then centrifuged at high speed (10,000 × g for 10 minutes at 4°C) to precipitate cellular debris. The supernatant was used for protein estimation and immunoblotting experiments. SVF-sEV isolates were kept on ice throughout the process. The protein concentration was measured using a commercially available Pierce™ Enhanced BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. Absorbance at 562 nm was measured using a Thermo Fisher Scientific Multiskan microplate reader.
Proteins were separated by SDS-PAGE electrophoresis and transferred to a PVDF (Immobilon) membrane. The membranes were probed with specific antibodies, and the expression of a housekeeping protein was confirmed using appropriate secondary antibodies. We probed for the expression of key EV-specific markers: TSG-101 (involved in multivesicular body synthesis), CD63 (involved in EV formation), and GRP94 (a negative marker used to confirm the enrichment of sEVs in the sample)3233.
Primary antibodies were used at a dilution of 1:1000 and secondary antibodies at 1:10,000. The antibodies used were rabbit polyclonal anti-CD63 (catalog no. SAB4301607, Invitrogen), anti-TSG101 (catalog no. PA531260, Invitrogen), anti-GRP94 (catalog no. SAB2101094, Invitrogen), and mouse monoclonal anti-β-actin (catalog no. MA1140, Invitrogen). All horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit polyclonal G21234, goat anti-mouse polyclonal G21040) were from Thermo Fisher Scientific. The specific positive sEV markers (CD63 and TSG101) and the negative marker (GRP94) were detected by immunoblotting using Bio-Rad's Western ECL Substrate.
Results
Identification of SVF cells isolated by collagenase type II vs. type IV
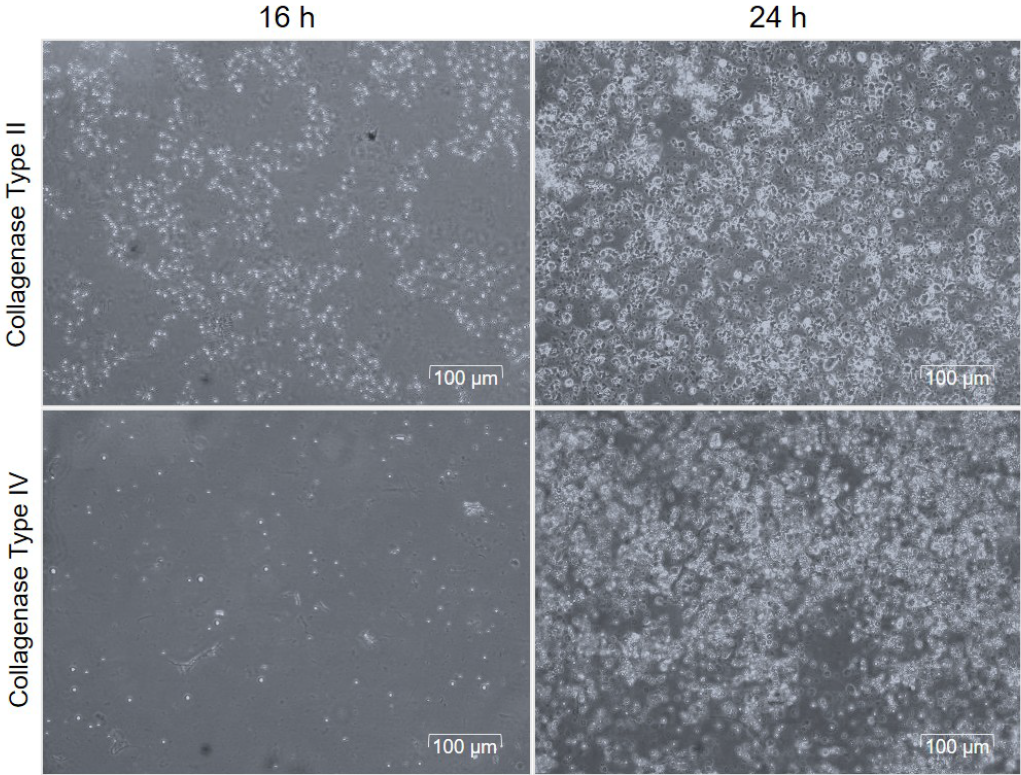
The quantification of viable SVF cells is essential for enhancing the yield of SVF-sEVs. To assess both cell count and viability, we employed the Trypan Blue exclusion assay, a reliable method for distinguishing living from dead cells on the basis of membrane integrity. The results (Figure 1) show SVF cells prepared with collagenase II and collagenase IV at two time points, highlighting differences in cell viability and integrity.
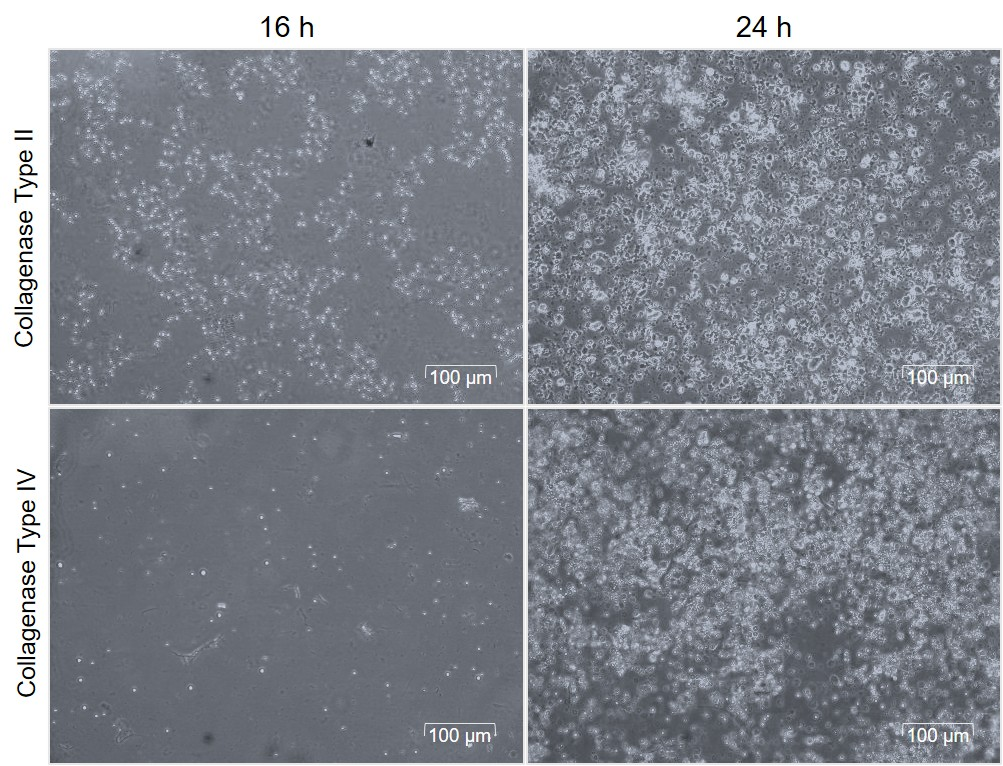
Cell count and viability of SVF cells following the tissue digestion with collagenase type II and collagenase type IV enzymes.
Quality validation of SVF-sEVs
NTA for particle-size distribution and concentration
Particle concentrations of samples digested with collagenase II were compared with those treated with collagenase IV. The mean sEV diameter was ~120 nm; volume- and dilution-corrected values are presented in Figure 2i–vi and summarized in Figure 3.
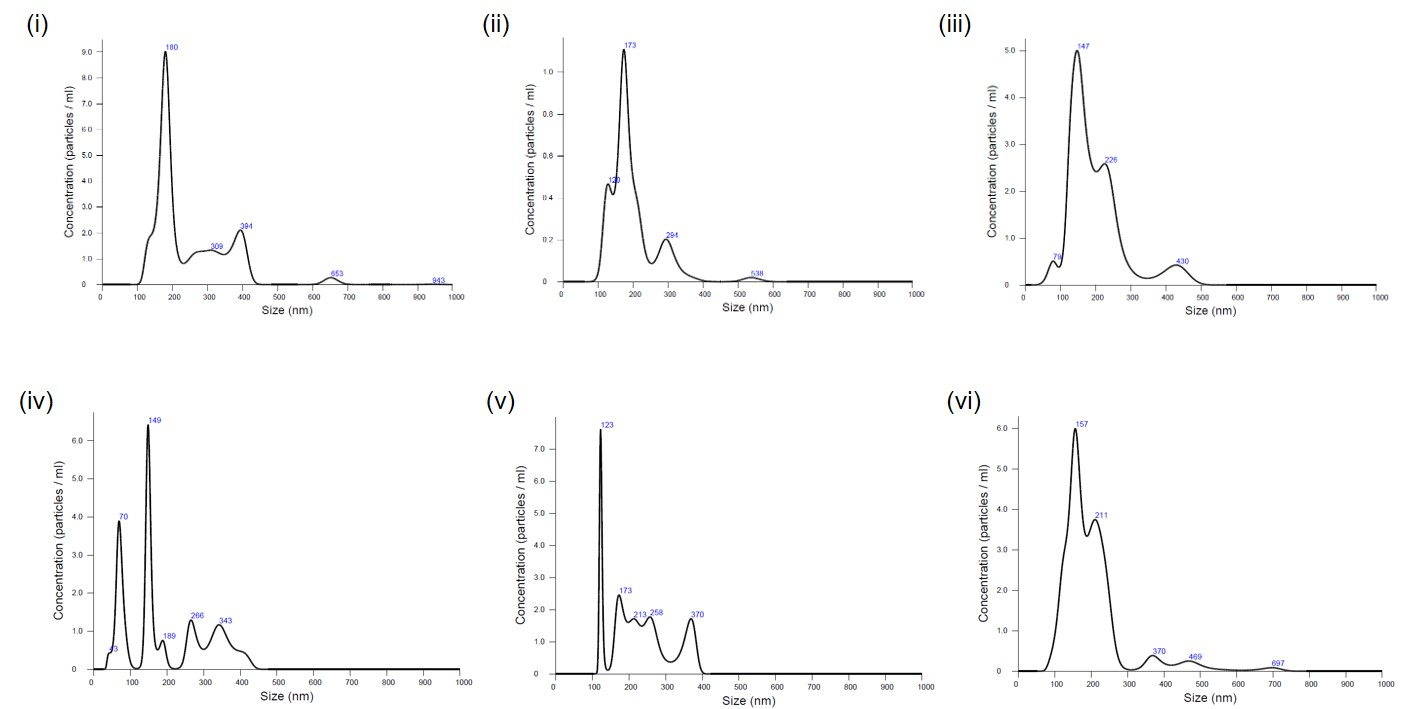
Nanoparticle tracking analysis (NTA) of isolated SVF-sEVs.
SVF-sEVs Concentration – (i) to (iii); Collagenase type II and (iv) to (vi) Collagenase type IV. (i) 37.1 particles/ frame; (ii) 46.6 particles/ frame; (iii) 30.2 particles/frame.
(iv) 20.3 particles/ frame (v) 20.7 particles/frame (vi) 33.7 particles/ frame.
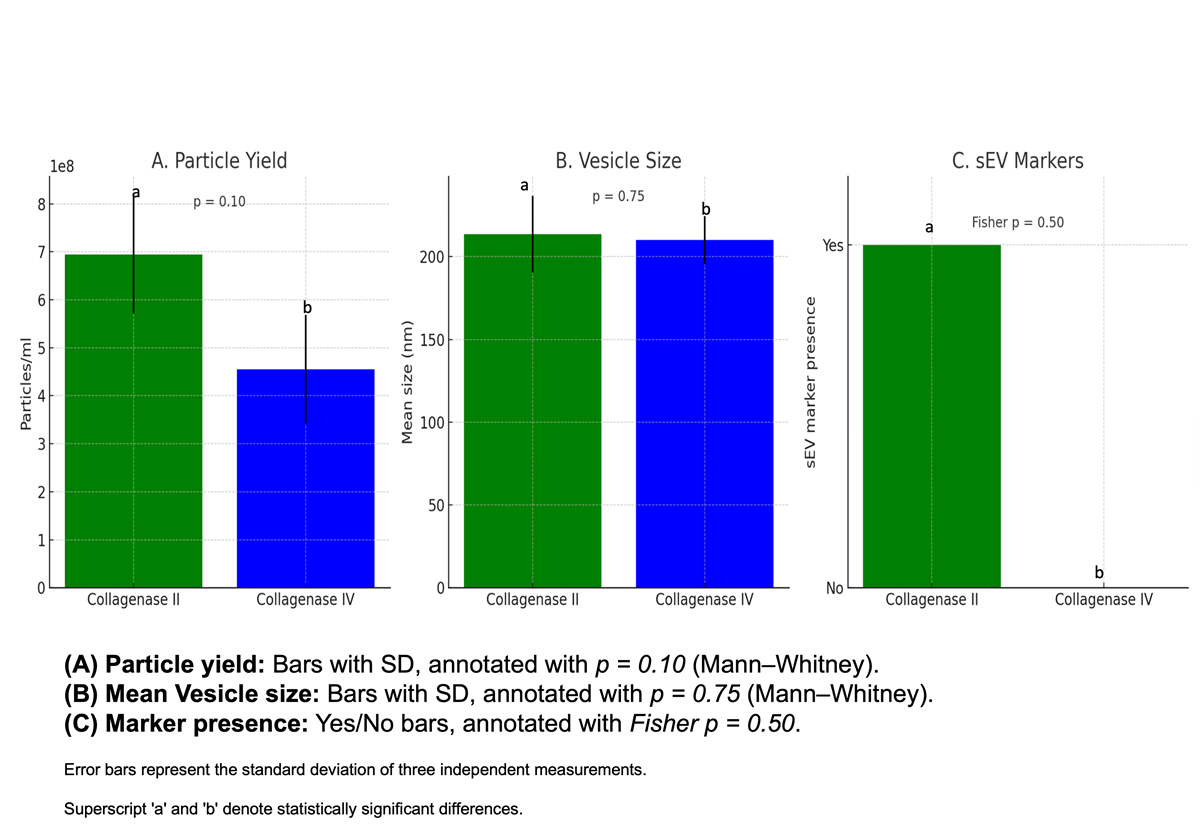
Collagenase type II improves yield and marker retention of sEVs from hHAT-SVF . When comparing enzymatic digestion methods, collagenase type II consistently yielded a higher concentration of small extracellular vesicles (sEVs) compared to collagenase type IV. A Mann–Whitney U test indicated a trend towards increased particle concentration with type II (U = 8.0, p = 0.10). Vesicle size distribution was similar between the two groups (U = 3.5, p = 0.75). Importantly, the presence of established sEVs markers was detected only in vesicles isolated using collagenase type II, whereas collagenase type IV–derived vesicles lacked these markers. Fisher’s Exact Test supported this observation (odds ratio = ∞, p = 0.5). Together, these findings suggest that collagenase type II is superior to type IV for isolating high-quality sEVs fromhHAT-SVF, providing both higher particle yield and positive sEVs marker expression. While statistical confirmation is limited by sample size, the biological relevance and consistency across replicates strongly favour collagenase type II for future SVF-sEVs isolation studies.
NTA confirmed the presence of sEVs in the intermediate fat layer, which showed a profile similar to that of the SVF pellet. In contrast, no sEVs were detected in the supernatants from either collagenase treatment, indicating that vesicles were not lost during enzymatic digestion. ζ-potential (ZP) analysis confirmed their surface charge. These datasets are available on request.
TEM and FM for ultrastructural visualization
Transmission-electron-microscopy (TEM) and fluorescence-microscopy (FM) images confirmed the intact cup-shaped morphology and protein content of SVF-sEVs isolated with collagenase II (Figure 4i–iv).
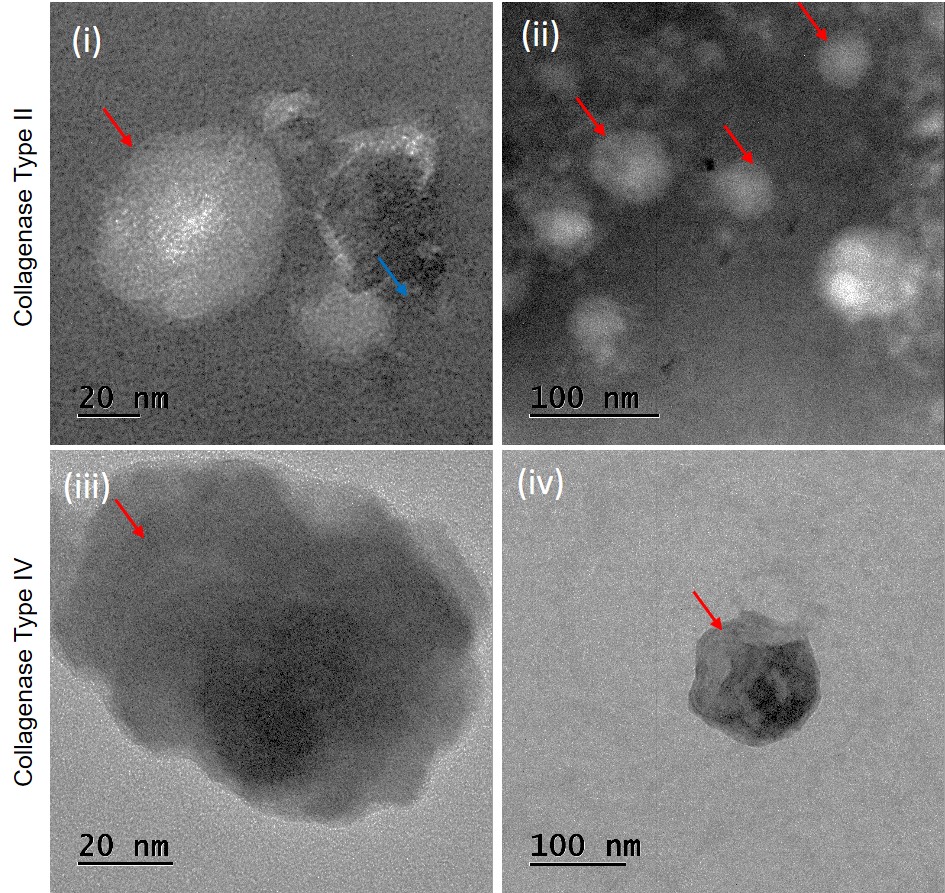
Images of transmission electron microscopy (TEM) of SVF-sEVs. (i) & (ii)- Collagenase type II: Red arrows indicate intact sEVs. Blue arrow shows lipoprotein aggregates. (iii) & (iv)- Collagenase type IV: Red arrows indicate ruptured sEVs.
WB for EV-marker identification and protein quantification
Western-blot analysis compared SVF-sEV preparations obtained with the two collagenases. Collagenase II yielded more SVF-sEVs, which were positive for CD63 and TSG101 but negative for GRP94 (Figure 5a–c), confirming their endosomal origin and the absence of endoplasmic-reticulum contamination in accordance with MISEV guidelines. In contrast, collagenase IV produced fewer vesicles and weaker antigen signals, consistent with its lower yield and purity.
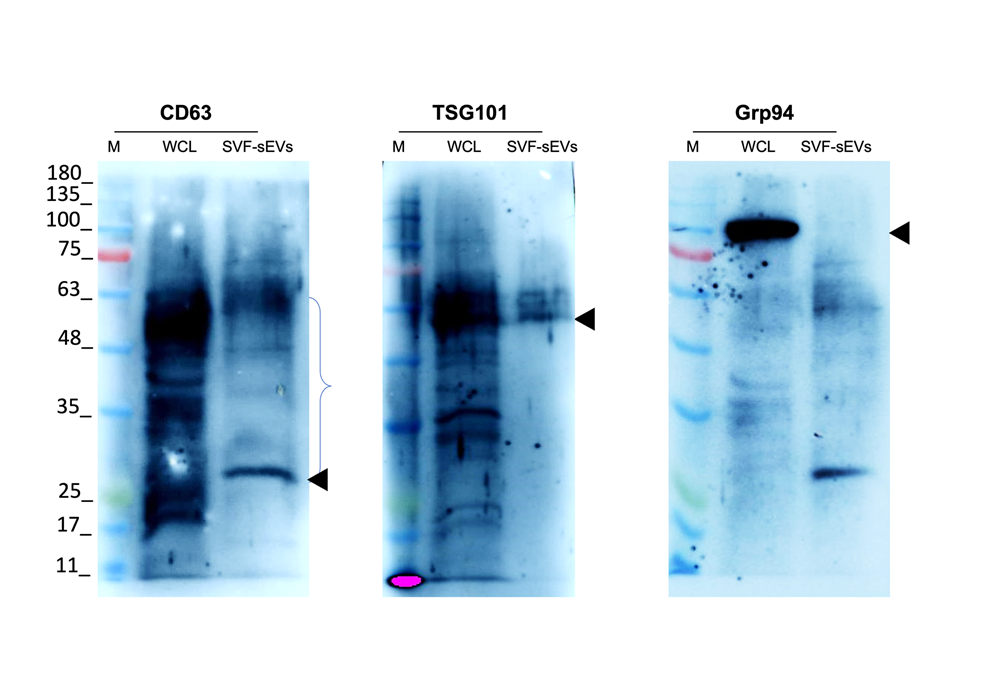
Detection of sEV-specific markers by Western blot. CD63 and TSG101-Positive markers. Grp94- Negative marker . Abbreviations: * M: Marker; WCL: Whole cell lysate; SVF-sEVs: Stromal vascular fraction-derived small extracellular vesicles.
|
Aspect |
Current Challenge |
Proposed Approach |
|
ti-sEVs isolation |
ECM entraps vesicles; low yield |
Enzymatic ECM breakdown using collagenase |
|
Vesicle purity |
Contamination from ruptured cells |
Optimized digestion to preserve cell integrity |
|
Tissue source |
Ethical and invasive procurement issues |
Use of accessible, minimally invasive hHAT |
|
Protocol efficiency |
Multi-step, low-throughput processes |
One-step, cost-effective isolation strategy |
|
Application potential |
Limited due to inconsistent quality of ti-sEVs |
Consistent high-yield isolation from SVF-sEVs |
Discussion
Small extracellular vesicles (sEVs) have emerged as a key therapeutic strategy for addressing tissue damage from surgery, trauma, and various disorders. Their therapeutic potential is largely due to the stromal vascular fraction (SVF), which contains lipid-laden adipocytes, blood vessels, and a collagenous extracellular matrix (ECM). These sEVs mirror many of the biological properties of their parent human adipose tissue (hAT) cells, enhancing their effectiveness in the biomedical field.
The method employed to isolate sEVs from the SVF (SVF-sEVs) significantly influences their overall quality and therapeutic efficacy; different isolation techniques yield variations in composition between the resulting fractions. For instance, the pellet contains SVF cells and debris, while the floating middle-fat layer holds cells that share similarities with those in the pellet, along with cell-ECM adhesions3334353637. Understanding these nuances is essential for optimizing the yield and quality of SVF-sEVs, ultimately enhancing their therapeutic outcomes.
To minimize the loss of a small or invisible SVF pellet during sample handling and to enhance the release of SVF-sEVs embedded in the ECM, we processed a larger volume of initial human adipose tissue (hAT) sample. This approach provided a minimally invasive and ethically sound alternative, improving the practicality of obtaining tissue-derived sEVs. We included non-obese young adults with a healthy BMI and selected liposuction aspirates from 3600 donor sites using the protocol by Priglinger.39.
To identify conditions that were mild enough to preserve the sEVs while removing ECM, cells, and lipids (debris), we used enzymatic tissue digestion. The results of this study indicate that using collagenase type II yielded a clear SVF pellet with minimal residual fat. The most effective conditions were determined to be 0.1% type II collagenase, incubated at 37°C for 4.5 hours with moderate shaking at 35 rpm. In contrast, using type IV collagenase under the same conditions led to incomplete tissue digestion, leaving a middle-fat layer above the SVF pellet and reducing vesicle recovery.
To ensure both yield and purity, we added a protease inhibitor during sonication to prevent degradation by endogenous proteases. Afterwards, the protease activity was deactivated by incubating the sample at 60°C for 10 minutes to prevent interference with downstream applications. This study also demonstrated that, regarding tissue processing, issues such as over-freezing, repeated freeze-thaw cycles, and prolonged incubation of SVF-conditioned media can negatively impact results. Regarding cellular condition, collecting SVF cells during the log phase of cell growth (16–20 h post-seeding), when they reach approximately 50% confluency, results in a higher yield. Furthermore, collecting sEVs from two distinct time points in culture further optimizes the total yield. For culture setup, seeding >200,000 SVF cells in T75 flasks allows for larger batches, further increasing both the yield and the quality. Finally, storing the freshly prepared SVF-sEVs in DPBS at –80°C in 1 mL aliquots helps reduce degradation. Our results also indicate that the role of differential ultracentrifugation (DUC) and volume scaling addresses lipid-based vesicle loss. When combined with a simplified one-step sEV isolation technique called DUC, we enhanced the retention of intact SVF-sEVs, thereby improving efficiency and scalability compared to traditional multi-step methods. DUC at 200,000 × g for >90 min promotes ectosome pelleting and reduces total protein concentration. Conversely, DUC at 200,000 × g for <70 min results in insufficient yield of SVF-sEVs and compromises purity. The optimal approach is to conduct two rounds of DUC at 200,000 × g for 70 minutes each at 4°C, with a PBS wash in between. Despite advancements in the DUC protocol, which relies on particle density and centrifugal force, there is still a risk of non-EV aggregates. Such aggregates can lead to contamination due to patient variability and several factors, including sample conditions (type, quality, and volume), g-force, duration, rotor type, and the number of spins404142434445.
To address this, we introduced an ultrafiltration (UF) step. We used Amicon ultrafiltration to concentrate the diluted SVF-sEV isolates after the two DUC steps. Combined with a MISEV-based validation of the SVF-sEVs characterization, these strategies collectively facilitated a consistent and high-yield isolation of SVF-sEVs across donors. This approach effectively minimized variability and aggregation artefacts, such as retention of buoyant sEVs that would otherwise rise to the surface due to their high neutral lipid or fatty acid content. These results are supported by findings in the nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), fluorescence microscopy (FM), and western blot (WB) images. Assessment of NTA data showed that the average concentration of sEVs from the six SVF samples examined in this study demonstrated improved quality and yield with a concentration of 6.94 × 10 particles/mL for type II collagenase, compared to type IV collagenase, which yielded 4.55 × 10 particles/mL. Assessment of TEM images of sEVs isolated with type II collagenase revealed intact spherical structures with a visible lipid bilayer membrane. Additionally, some lipoprotein-like structures lacking visible membranes, measured up to 400 nm, which is larger than what is typically observed in lipoaspirate samples. The samples also contained smaller vesicles ranging from 20 to 200 nm, indicating structural heterogeneity that aligns with the NTA data and polydispersity index. Some of the vesicles displayed spot-like features, suggesting electron-dense cargo; however, multivesicular bodies were not observed, contrary to other published reports. In contrast, sEVs isolated with type IV collagenase exhibited non-spherical particles larger than 400 nm, indicating possible rupture and protein leakage. This issue is common in stored sEVs due to degradation or stress from an improperly tuned mechanical digestion process, consistent with the conditions established for this sample. Furthermore, more vesicles in these samples showed low contrast and punctate membranes, hinting at membrane damage and suggesting that SVF-sEVs may have ruptured, causing the protein contents to spill out. This observation is further supported by the final protein characterization. SVF-sEVs treated with type II collagenase displayed improved quality, suggesting a wider range of potential applications in therapeutic settings.
In summary, this study offers scalable and reproducible workflows, making it time-efficient by reducing multi-step purification processes. Furthermore, the findings offer a practical pathway from lab research to patient care. These results provide a basis for developing therapeutic strategies and exploring novel interventions for antibiotic-resistant microorganisms (ARM).
Conclusions
In summary, this study presents an optimized strategy to overcome the major challenges of tissue-derived small-EV (ti-sEV) isolation. By employing type II collagenase for human healthy adipose tissue (hHAT), we achieved more efficient tissue dissociation, reduced vesicle entrapment, and preserved sEV surface integrity, collectively increasing both yield and purity. In addition, integrating a dual time-point stromal vascular fraction (SVF) collection with a two-step DUC + UF workflow markedly improved SVF-sEV recovery compared with previous adipose-EV studies that relied on single harvests or less efficient methods. These findings lay the groundwork for evaluating SVF-sEV efficacy in clinical settings and are particularly relevant to the emerging field of SVF-sEV-based nanomedicine. Unlike earlier reports that tested collagenases separately, our study directly compares type II and type IV enzymes for EV isolation from human adipose-derived SVF; type II collagenase produced higher-quality EVs under identical conditions, underscoring enzymatic differences and offering new guidelines for improved EV isolation. Cost-wise, type II collagenase is inexpensive relative to other high-cost methods aimed at boosting yield and purity, rendering the protocol both efficient and economical.
Additional in-vivo studies are needed to validate the reliability and therapeutic efficacy of this approach before clinical translation. Such validation will also guide dose-dependent nanotherapy under real-world conditions, where manual SVF-sEV isolation may be necessary because of logistical, economic, or operational constraints.
Abbreviations
ARM: antibiotic-resistant microorganisms; BCA: Bicinchoninic Acid assay; BMI: Body Mass Index; CEC: Central Ethics Committee; DAPI: 4',6-diamidino-2-phenylindole; DMEM: Dulbecco’s Modified Eagle Medium; DPBS: Dulbecco's Phosphate-Buffered Saline; DUC: differential ultracentrifugation; ECL: Enhanced Chemiluminescence; ECM: extracellular matrix; EVs: Extracellular vesicles; FBS: Fetal Bovine Serum; FM: fluorescence microscopy; fps: frames per second; g: relative centrifugal force; GMP/GCP: Good Manufacturing Practice/Good Clinical Practice; h: hour(s); hHAT: healthy human adipose tissue; HRP: Horseradish Peroxidase; kDa: kilodalton; keV: kiloelectronvolt; kg m⁻²: kilograms per square meter; min: minute(s); MISEV: Minimal Information for Studies of Extracellular Vesicles; mL: milliliter; nm: nanometer; NIST: National Institute of Standards and Technology; NTA: nanoparticle tracking analysis; PBS: phosphate-buffered saline; PS: penicillin/streptomycin; PVDF: Polyvinylidene Fluoride; RIPA: Radioimmunoprecipitation assay buffer; rpm: rotations per minute; s: second(s); SDS-PAGE: Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis; sEVs: small extracellular vesicles; SVF: stromal vascular fraction; SVF-sEVs: stromal vascular fraction-derived small extracellular vesicles; TDEs: tissue-digestion enzymes; TEM: transmission electron microscopy; ti-sEVs: tissue-derived small extracellular vesicles; UF: ultrafiltration; µL: microliter; WB: western blotting; ZP: Zeta potential; °C: degrees Celsius.
Acknowledgments
The authors would like to acknowledge the support of Nitte (Deemed to be University) in carrying out this study.
Author’s contributions
Original draft preparation: Dr. VMHS; Formal analysis: Dr. VMHS, Dr. NSS, Dr. DU, Ms. NNK; Editing & analysis: Dr. VMHS, Dr. AVS; Writing – review and editing: Dr. VMHS, Dr. MKB, Dr. PP, Dr. NSS, Dr. AVS. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki (2004). Approval was granted by the local central Ethics Committee (Date: Jan 13 2022 / No. NU/CEC/2021/230).
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

