The effect of cannabinoids on liver injury associated with acute pancreatitis
- Department of Medical Pharmacology, Faculty of Medicine, Zonguldak Bulent Ecevit University, Zonguldak, Kozlu, 67600, Turkey
- Department of Medical Pathology, Faculty of Medicine, Zonguldak Bulent Ecevit University, Zonguldak, Kozlu, 67600, Turkey
- Department of Biostatistics, Faculty of Medicine, Zonguldak Bulent Ecevit University, Zonguldak, Kozlu, 67600, Turkey
Abstract
Introduction: Acute pancreatitis (AP), an inflammatory condition, can lead to serious complications such as systemic inflammatory response syndrome (SIRS) and multiple organ failure. The liver is one of the organs affected during this process. Cannabinoids have demonstrated efficacy in the treatment of numerous pathologies, including pain, inflammation, and gastrointestinal diseases. In this study, we evaluated the effects of the phytocannabinoid cannabidiol (CBD) on AP-associated liver injury (LI).
Methods: Rats were randomized into control, AP, and AP+CBD groups. The AP and AP+CBD groups received caerulein (50 µg/kg; five intraperitoneal injections), while the AP+CBD group additionally received prophylactic CBD (10 mg/kg). Pancreatic and liver tissues were evaluated histopathologically, immunohistochemically, and biochemically, and serum lipase levels were measured.
Results: Caerulein administration increased serum lipase levels and induced tissue damage by elevating oxidative stress and nuclear factor-κB (NF-κB) immunoreactivity in the pancreas and liver. CBD treatment reduced histopathologic damage and oxidative stress in both organs. NF-κB immunoreactivity in the pancreas was significantly reduced, while its activity in the liver appeared to be elevated.
Conclusion: Our study suggests that CBD possesses therapeutic potential in AP by reducing oxidative stress and NF-κB activity. In the liver, CBD may mitigate AP-associated LI by lowering oxidative stress and differentially modulating NF-κB activity through as-yet-undefined mechanisms. Additional research is needed to clarify CBD’s hepatic effects in the context of AP.
Introduction
Acute pancreatitis (AP) is an inflammatory condition characterized by pain in the upper abdomen, nausea, vomiting, and elevated serum lipase levels. It usually follows a mild course, but in 15–20% of cases local or systemic complications can occur. These cases frequently lead to multiple-organ failure (heart, lungs, liver, and other organs) and ultimately death1. Therefore, besides treating pancreatic pathology, preventing remote organ damage is essential to reduce mortality.
Cannabinoids comprise phytocannabinoids found in the cannabis plant, endocannabinoids synthesized in the body from arachidonic acid, and laboratory-made synthetic cannabinoids. The two principal plant-derived constituents are cannabidiol (CBD) and Δ⁹-tetrahydrocannabinol (THC). Among endocannabinoids, 2-arachidonoylglycerol and anandamide (AEA) are the most prominent2. Cannabinoids have shown therapeutic potential against pain, inflammation, cancer, neurodegenerative diseases, and gastrointestinal disorders3. In recent years studies have examined their effects on pancreatic damage45; however, their impact on AP-induced liver injury (LI) remains unclear.
Nuclear factor-κB (NF-κB) is an inducible transcription factor that regulates the immune system and drives inflammatory responses6. Dysregulated NF-κB signaling has been linked to numerous diseases, including inflammatory and autoimmune disorders, cancer, and cardiovascular disease7. Another key driver of inflammation and tissue damage is oxidative stress, which also contributes to pancreatic and liver injury89.
Methods
Animal Model
Adult female Wistar albino rats weighing 200–300 g were used in the study. The rats were randomly classified into three groups (
Experimental groups
|
Group |
Number of animals |
Chemicals |
Application method |
|
Control |
n=10 |
- |
- |
|
AP |
n=10 |
Caerulein (60 μg/kg) |
i.p. |
|
AP+CBD |
n=8* |
CBD (10 mg/kg)/Caerulein (60 μg/kg) |
i.p./i.p. |
The rats were maintained at room temperature (22 °C) under a 12-h light/12-h dark cycle and provided with unrestricted access to drinking water and pellet feed containing 21 % crude protein. The experimental protocols complied with our institutional guidelines, which are consistent with the “Guide for the Care and Use of Laboratory Animals (US National Institutes of Health, revised 1996).” Approval was received from the Zonguldak Bulent Ecevit University Experimental Animal Ethics Committee (Protocol/Approval Number: 2021-30-02/12; Approval Date: 02 December 2021).
No treatment was applied to the control group. To induce the acute pancreatitis (AP) model, the AP and AP+CBD groups received caerulein five times at 1-h intervals. In the CBD group, CBD was administered 30 min before the first caerulein injection.
Six hours after the last caerulein injection, all animals were sedated with ketamine (75 mg kg⁻¹, i.p.) and xylazine (7 mg kg⁻¹, i.m.). Blood samples were collected from the abdominal aorta, after which the aorta was cut and the animals were euthanised. The pancreas and liver were then removed. The duodenal region of the pancreas and the left lateral lobe of the liver were preserved in formalin for histopathological and immunohistochemical analyses, whereas the splenic region of the pancreas and the median lobe of the liver were stored at −80 °C for biochemical analyses.
Histopathologic Examination
Tissue samples were fixed in 10 % formalin, dehydrated in ethanol, cleared with xylene, and embedded in paraffin. Sections 5 µm thick were cut and stained with hematoxylin–eosin. Pancreatic tissues were evaluated for acinar hypertrophy, autophagy, and necrosis; liver tissues were evaluated for portal-area inflammation and spotty necrosis.
Immunohistochemical Examination
Cell Signaling Technology (CST) NF-κB (D14E12) antibody, cat. no. 8242S (CST, Danvers, MA, USA), was diluted 1:300 and incubated for 1 h. Antigen retrieval was performed with the Cell Conditioning 1 (CC1) EDTA-based protocol (Roche) for 64 min. The Roche UltraView Universal DAB Detection Kit was used for secondary detection, and imaging was performed according to the standard UltraView IHC protocol. All procedures were carried out with a Ventana BenchMark XT IHC stainer (Roche/Ventana, Tucson, AZ, USA).
Biochemical Analysis
Serum lipase activity was measured, and malondialdehyde (MDA) and glutathione (GSH) levels together with superoxide dismutase (SOD) and catalase (CAT) activities were analysed in pancreatic and hepatic tissues.
Determination of Serum Lipase Activity
The reaction was initiated by adding R1 (buffer/colipase/colate) and R2 (emulsion/chromogenic substrate/colate) to the sample. Lipase cleaves the chromogenic substrate, producing glutaric acid and methylresorufin. The red colour formed in the alkaline solution was measured photometrically to determine lipase activity.
Determination of MDA Levels (nmol L⁻¹)
Tissue samples were treated with 10 % trichloroacetic acid to precipitate proteins and then centrifuged. The supernatant was reacted with 0.67 % thiobarbituric acid in a boiling water bath for 10 min. After cooling, MDA was quantified by measuring the absorbance at 532 nm.
Determination of SOD Activity
In this assay, superoxide radicals generated by the reaction of xanthine and xanthine oxidase react with 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride to form a red formazan dye. SOD activity is determined from the degree of inhibition of dye formation.
Determination of CAT Activity
CAT converts hydrogen peroxide into water and oxygen. When ammonium molybdate is added, the reaction stops and hydrogen peroxide reacts with ammonium molybdate. CAT activity is determined by photometrically measuring the yellow complex formed at 405 nm.
Determination of GSH Levels (mg L⁻¹)
GSH levels were measured with a Rat GSH ELISA Kit (EA0113Ra, BT-Lab, Shanghai, China) according to the manufacturer’s instructions.
Chemicals
Caerulein (≥90 %, 2.5 mg, C070000) was purchased from Toronto Research Chemicals (Ontario, Canada). CBD (≥98 %, 10 mg, 90080) was obtained from Cayman Chemical (Ann Arbor, MI, USA). Ketamine (Ketalar, 500 mg 10 mL⁻¹; Pfizer, USA), xylazine (Xylazinbio 2 %; Bioveta, Czech Republic), and methanol (solvent for caerulein) were also used.
Sample-Size Calculation
In calculating the sample size, the large effect size defined by Cohen (1988)10 was applied. For the Kruskal–Wallis test, an effect size of 0.80 required 18 animals (six per group) to attain 70 % power at the 95 % confidence level. The calculation was performed with G*Power 3.1.9.2.
Data Analysis and Statistics
Data were analysed with SPSS 29.0 (IBM Corp., Armonk, NY, USA). Quantitative variables are presented as median (minimum–maximum). The Kruskal–Wallis test was applied for non-parametric multi-group comparisons. Pairwise differences were assessed with the Mann–Whitney U test using the Bonferroni correction. Categorical variables were evaluated with the Fisher–Freeman–Halton χ² test. In all analyses, p < 0.05 was considered statistically significant.
For effect-size calculations, the epsilon square (ε²) coefficient was used for the Kruskal-Wallis test, and Cramér’s V coefficient was used for the chi-square test. The ε² coefficient was interpreted using the classification proposed by Tomczak and Tomczak (2014). According to this classification, values between 0.01 and 0.08 indicate a small effect size, values between 0.09 and 0.24 indicate a medium effect size11, and values of 0.25 and above indicate a large effect size. When interpreting Cramér's V coefficient, the classification proposed by Cohen (1988) was used. According to this classification, values between 0.1 and 0.29 are considered small effect size, values between 0.30 and 0.49 are considered medium effect size, and values of 0.50 and above are considered large effect size10.
Results
Effects of CBD on Serum Lipase Levels and Oxidative Stress
Caerulein administration caused a significant increase in serum lipase activity; however, CBD treatment failed to suppress this increase (
Comparative analysis of serum lipase levels and oxidative stress markers in pancreatic and liver tissues between experimental groups
|
Control (n=10) |
AP (n=10) |
AP+CBD (n=8) |
H (χ²KW) |
p-value |
Epsilon Square (ε²) |
Effect Size Interpretation | |
|
Median(Min/Max) |
Median(Min/Max) |
Median(Min/Max) | |||||
|
Serum lipase |
6.05(4.00/19.10) |
417.20(298.00/478.00)a |
468.30(400.00/497.00)a |
19.107 |
0.000 |
0.590 |
Large |
|
Pancreas MDA |
45.23(28.34/64.55) |
52.30(33.23/68.34) |
31.11(12.63/54.82) |
4.699 |
0.095 |
0.093 |
Medium |
|
Pancreas SOD |
78.40(38.00/85.40) |
112.90(72.20/136.30)a |
193.35(137.20/218.50)a,b |
13.752 |
0.001 |
0.405 |
Large |
|
Pancreas CAT |
83.00(55.00/112.50) |
82.50(61.00/123.50) |
48.00(35.00/102.50)a,b |
5.603 |
0.061 |
0.124 |
Medium |
|
Pancreas GSH |
1457.83(1207.19/1886.67) |
1278.36(1111.15/1507.30)a |
1252.88(1135.72/1375.90)a |
6.747 |
0.034 |
0.168 |
Medium |
|
Liver MDA |
51.86(39.66/57.30) |
47.34(31.90/66.85) |
18.72(9.59/42.71)a,b |
10.723 |
0.005 |
0.301 |
Large |
|
Liver SOD |
116.80(63.00/186.00) |
93.80(69.50/180.60) |
89.40(44.00/111.00) |
5.037 |
0.081 |
0.105 |
Medium |
|
Liver CAT |
61.00(55.00/83.00) |
68.00(41.00/82.50) |
110.50(63.00/129.50)a,b |
7.505 |
0.023 |
0.190 |
Medium |
|
Liver GSH |
1178.08(830.53/1332.12) |
1053.52(705.85/1365.41) |
1185.86(1037.36/1394.90) |
1.097 |
0.578 |
0.004 |
Negligible |
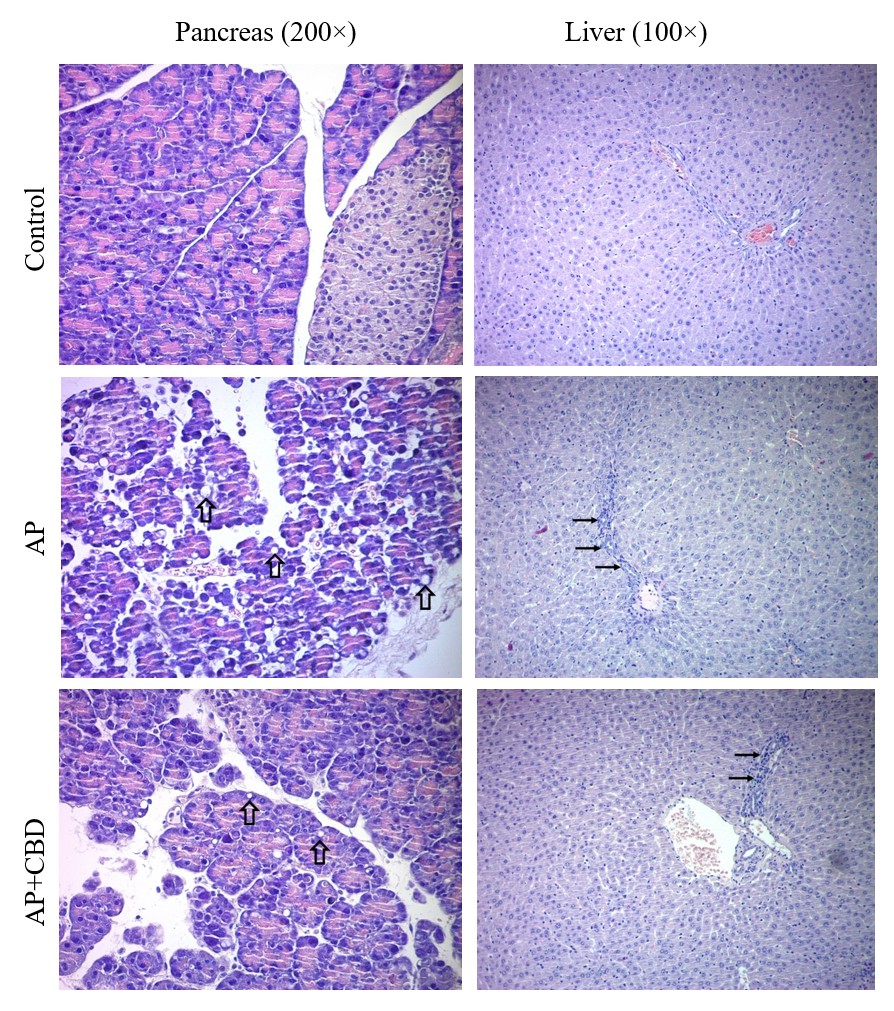
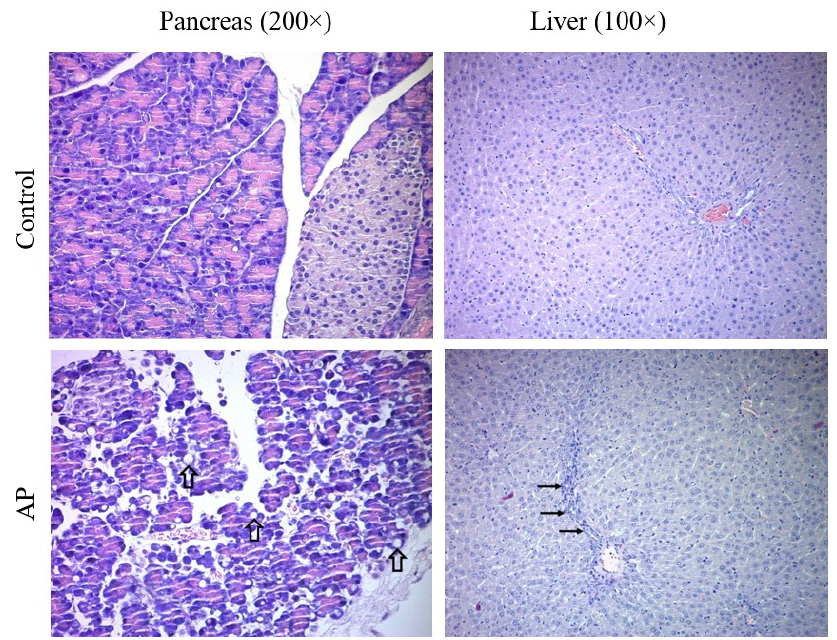
Rat pancreatic and liver tissues, hematoxylin-eosin staining results. Control group, pancreatic tissue with well-structured acini and islet of Langerhans and normal liver tissue. AP group pancreatic tissue, hypertrophic acini containing medium and small-sized cytoplasmic vesicles (thick arrows). Liver tissue, regenerative changes in hepatocytes and moderate inflammation in the portal area (thin arrows). AP+CBD group pancreatic tissue, acinar cells with small cytoplasmic vesicles (thick arrows). Liver tissue, mild inflammation in the portal area (thin arrows) and sinusoidal dilation. Abbreviations: AP: acute pancreatitis, AP+CBD: acute pancreatitis+cannabidiol
Effects of CBD on Tissue Injury
After caerulein application, the pancreas was macroscopically edematous. Microscopic examination revealed hypertrophic acini containing small- to medium-sized cytoplasmic vesicles. CBD reduced the size of these cytoplasmic vesicles (Figure 1) Cramér’s V = 0.74, large effect size) and autophagy (χ² = 37.178, p < 0.001; Cramér’s V = 0.83, large effect size) were markedly increased. CBD significantly reduced acinar autophagy. A slight increase was also observed in acinar necrosis, and CBD reduced this increase as well (χ² = 6.565, p = 0.073). However, these changes were not statistically significant. Nevertheless, the Cramér’s V value was calculated as 0.35, indicating a medium effect size and suggesting that the observed changes may be biologically significant.
Histopathological evaluation revealed regenerative changes in hepatocytes and moderate inflammatory infiltration in the portal area. CBD decreased the severity of these histopathological findings (Figure 1). Specifically, portal-area inflammation (χ² = 8.986, p = 0.027; Cramér’s V = 0.40, medium effect size) and spotty necrosis (χ² = 6.332, p = 0.024; Cramér’s V = 0.48, medium effect size) were significantly increased. These increases were significantly reduced by CBD.
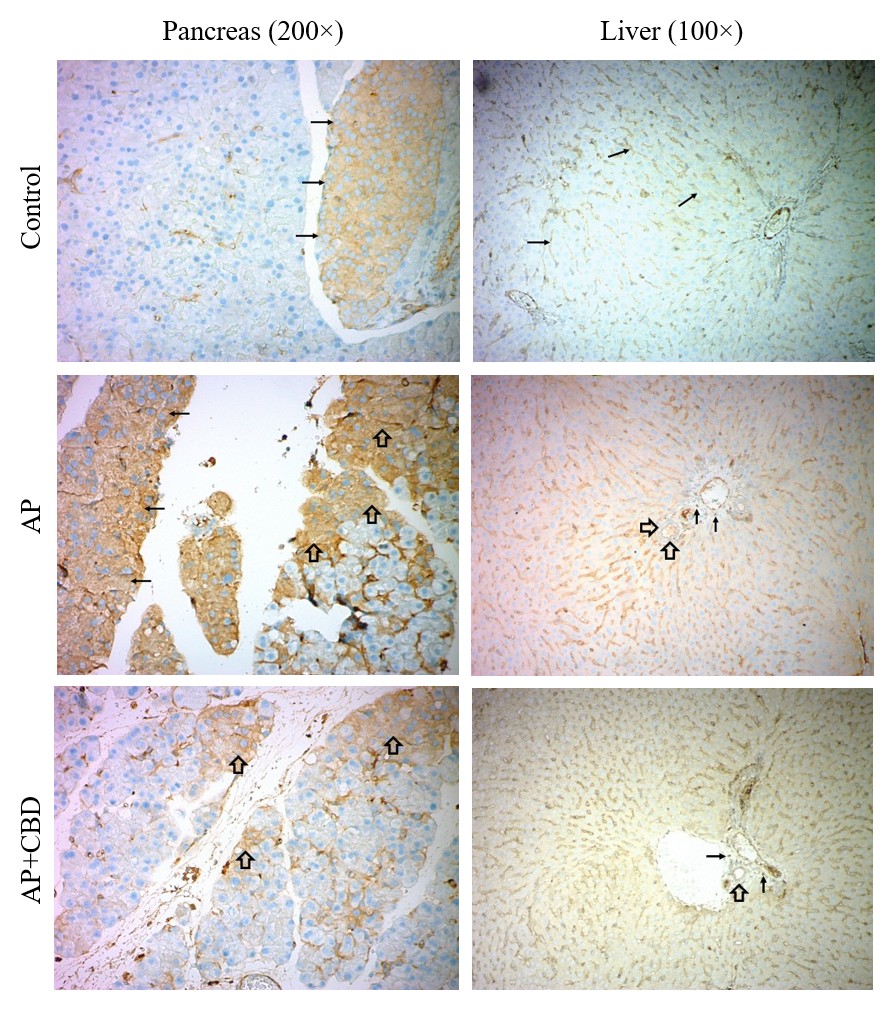
Rat pancreatic and liver tissues, nuclear factor-kappaB (NF-κB) immunohistochemistry staining results. Control group, NF-κB staining is observed in the Langerhans islet cells (thin arrows) in the pancreatic tissue. Liver tissue, staining is observed in Kupffer cells (thin arrows). AP group, strong and diffuse staining is observed in the cytoplasm of acinar cells (thick arrows) and Langerhans islet cells (thin arrows) in the pancreatic tissue. Liver tissue, staining is observed in Kupffer cells as well as in the bile duct (thick arrows) and inflammatory cells (thin arrows). AP+CBD group, the staining observed in the acinar cells (thick arrows) regressed to local foci. Liver tissue, staining is observed in Kupffer cells as well as in the bile duct (thick arrow) and inflammatory cells (thin arrows). Staining in the bile duct is more intense in the AP group and milder in the AP+CBD group. Abbreviations: AP: acute pancreatitis, AP+CBD: acute pancreatitis+cannabidiol
Effects of CBD on NF-κB Expression
Strong and diffuse staining for NF-κB was observed in acinar cells. CBD reduced both the intensity and extent of this staining (Figure 2). The evaluation revealed a significant increase in the percentage of the NF-κB-positive area (χ² = 69.913, p = 0.026; Cramér’s V = 0.43, medium effect size), which was markedly reduced by CBD. A slight increase in NF-κB staining intensity was also observed, and this increase was diminished with CBD (χ² = 3.360, p = 0.521). However, these changes were not statistically significant. Nevertheless, the Cramér’s V value was calculated as 0.35, indicating a medium effect size and suggesting that the observed changes may be biologically significant.
NF-κB staining was observed in the bile-duct epithelium, inflammatory cells, and Kupffer cells. CBD reduced staining intensity in the bile duct while increasing it in other areas (Figure 2). The evaluation revealed a significant increase in the NF-κB-positive area (χ² = 8.625, p = 0.011; Cramér’s V = 0.56, large effect size) and staining intensity (χ² = 9.788, p = 0.016; Cramér’s V = 0.42, medium effect size). CBD further enhanced these increases.
Discussion
This study represents the first attempt to examine the actions of cannabinoids on acute pancreatitis (AP)–associated liver injury (LI). Our findings suggest that cannabidiol (CBD) may attenuate AP severity and remote organ damage through its antioxidant and anti-inflammatory effects.
AP is a clinical condition characterized by pancreatic inflammation ranging from mild edema to severe necrosis and by elevated serum lipase12. The inflammatory response that begins in the pancreas may extend systemically, leading to systemic inflammatory response syndrome and multiple organ failure. The organs most commonly affected are the lungs, heart, liver, and kidneys13.
In our study, caerulein administration significantly increased serum lipase levels, mirroring clinical observations. Within the pancreas, reduced glutathione (GSH) content, acinar hypertrophy and autophagy, and an increased percentage of NF-κB–positive cells with a strong, diffuse cytoplasmic signal were observed—findings consistent with AP. Secondary to pancreatitis, the liver exhibited regenerative hepatocyte changes, moderate portal inflammation, spotty necrosis, and an increased intensity and percentage of NF-κB immunoreactivity.
Oxidative stress arises from an imbalance between oxidants and antioxidants and damages cell membranes, proteins, and DNA. In AP, reactive oxygen-species production increases, causing significant injury to both the pancreas and liver. Malondialdehyde (MDA), a marker of lipid peroxidation, reflects this oxidative burden. Cells counteract oxidative stress with antioxidants such as GSH and with enzymes including catalase (CAT) and superoxide dismutase (SOD)14.
In the caerulein-induced AP model, carvacrol lowered hepatic MDA and oxidized deoxyguanosine while increasing SOD, CAT and glutathione-peroxidase activities, thereby attenuating inflammation and necrosis15. Similarly, in an L-arginine–induced model, tiron decreased MDA and NADPH-oxidase levels and elevated SOD and GSH, reducing AP-associated LI16. In our study, CBD likewise reduced hepatic MDA, increased pancreatic SOD, and elevated hepatic CAT.
NF-κB is not a single protein but a family of transcription factors (p50, p52, RelA/p65, RelB, c-Rel) that form homo- or heterodimers. The predominant p65:p50 heterodimer remains cytoplasmic, bound to inhibitor κB (IκB) proteins. Upon stimulation (e.g., cytokines, pathogens, oxidative stress), IκB is phosphorylated and degraded, allowing NF-κB to enter the nucleus and drive genes involved in inflammation, immunity, and cell survival. Activation occurs via a canonical (p50:p65) and an alternative (p52:RelB) pathway; the former mediates rapid inflammatory responses, whereas the latter governs lymphoid organogenesis and sustained inflammation5.
The NF-κB pathway plays a critical role in the pro-inflammatory response in AP17 and in AP-associated LI14. For example, in an L-arginine model, one-week administration of nilotinib reduced pancreatic NF-κB immunoreactivity and ameliorated pancreatic and liver injury18. Likewise, albiforin inhibited the p38-MAPK/NF-κB pathway and curtailed LI19. Consistent with these reports, our study showed that CBD decreased the percentage of NF-κB–positive pancreatic cells. In the liver, NF-κB activity declined in bile ducts but increased in parenchyma, suggesting a dynamic, time-dependent regulation.
It has been reported that the p38-MAPK/NF-κB pathway is especially active in Kupffer cells and drives AP-associated LI by increasing cytokine production20. Conversely, p65 NF-κB/Fas-ligand signalling can induce Kupffer-cell apoptosis and limit inflammation21. In concanavalin-A hepatitis, hepatocyte NF-κB activation lessened TNF-α-mediated apoptosis22. These findings imply that early NF-κB activation may protect tissue. Our observation of heightened parenchymal NF-κB after CBD may reflect such a protective, early-phase mechanism.
During fulminant hepatitis of pregnancy, NF-κB DNA binding increases but p50 homodimers dominate, whereas p65 is deficient, leading to immune dysregulation23. Similarly, CBD was shown to promote RelA (p65) binding and exert antitumoral effects in glioblastoma cells24. The CBD-induced rise in total hepatic NF-κB in our work may therefore involve selective p65 activation.
In AP-associated LI, hepatocyte injury, Kupffer-cell activation and cytokine release are central, whereas cholangiocytes are less affected1425. CBD’s suppression of NF-κB in bile ducts but enhancement in parenchyma may thus reflect differential cellular sensitivity.
CBD is non-psychoactive and generally safe. It can act as an antagonist at cannabinoid receptors, increase anandamide by blocking its re-uptake/hydrolysis, and modulate many non-cannabinoid targets26. Numerous studies show CBD protects tissues against oxidative stress and inflammation2728. Two reports examined CBD in pancreatic injury. In a caerulein model (50 µg kg⁻¹ × 6), CBD (0.5 mg kg⁻¹) given twice reduced pancreatic histopathology, plasma TNF-α/IL-6, intrapancreatic IL-6 and MPO activity. In lipopolysaccharide-induced injury, CBD (5 mg kg⁻¹) lessened pancreatic damage, lowered p53, and up-regulated SIRT1, PGC-1α, insulin and amylin. We similarly observed increased pancreatic SOD, reduced acinar autophagy, and a shift from diffuse to focal NF-κB staining after CBD.
Previous studies have not addressed dose- or time-dependence of CBD in AP-associated LI. In a rat sepsis model, CBD (2.5–10 mg kg⁻¹, i.p.) decreased hepatic thiobarbituric-acid-reactive substances (TBARS) in the acute phase, yet protein carbonyls fell only at 5 mg kg⁻¹ and rose at 10 mg kg⁻¹; chronic dosing for nine days reduced both markers at all doses29. These data suggest that acute biochemical responses vary with dose, whereas chronic CBD uniformly lowers oxidative stress.
In our study, CBD reduced hepatic MDA and raised CAT, while portal inflammation and spotty necrosis declined. Parenchymal NF-κB initially rose, a change that may subside over time as CBD exerts longer-term anti-inflammatory effects. Supporting this view, 21-day CBD (10 mg kg⁻¹ day⁻¹) attenuated liver edema, histopathology and NF-κB activation in congestive hepatopathy30. Likewise, six-week CBD (2–18 mg kg⁻¹ day⁻¹) prevented AST elevation at 2 & 18 mg, and reduced fibrosis and necrosis at 18 mg in cirrhotic rats31. These findings indicate broader tissue protection with higher or prolonged CBD dosing.
In light of these data, future work should explore multiple CBD doses and extended observation periods to delineate its acute versus chronic effects on inflammation and oxidative stress.
Our study has several limitations. First, only a single CBD dose was used; dose–response relationships remain unknown. Second, outcomes were assessed 6 h after the final caerulein injection; later time points may reveal different effects. We hope the present data will guide future, more comprehensive studies.
Conclusions
Our findings indicate that cannabidiol (CBD) may benefit acute pancreatitis (AP) and the associated liver injury (LI) by reducing oxidative stress and modulating NF-κB activation. Collectively, these results support CBD as a potential therapeutic agent for both conditions; however, additional studies are required to clarify its mechanisms of action and clinical efficacy. These findings also pave the way for further research into the therapeutic applications of cannabinoids in pancreatic and hepatic diseases.
Abbreviations
AP: Acute Pancreatitis; SIRS: Systemic Inflammatory Response Syndrome; CBD: Cannabidiol; LI: Liver Injury; NF-κB: Nuclear Factor-κB; MDA: Malondialdehyde; GSH: Glutathione; SOD: Superoxide Dismutase; CAT: Catalase; THC: Δ⁹-Tetrahydrocannabinol; AEA: Anandamide; CST: Cell Signaling Technology; CC1: Cell Conditioning 1; DAB: 3,3'-Diaminobenzidine; EDTA: Ethylenediaminetetraacetic Acid; TBARS: Thiobarbituric Acid Reactive Substances; TNF-α: Tumor Necrosis Factor-alpha; IL-6: Interleukin-6; MPO: Myeloperoxidase; SIRT1: Sirtuin 1; PGC-1α: Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha; AST: Aspartate Aminotransferase; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; MAPK: Mitogen-Activated Protein Kinase; i.p.: Intraperitoneal; i.m.: Intramuscular
Acknowledgments
We would like to thank Zonguldak Bulent Ecevit University Scientific Research Projects Coordination Unit for their support.
Author’s contributions
All authors contributed equally to the study, read the final version and approved its publication.
Funding
This study was supported by Zonguldak Bulent Ecevit University Scientific Research Projects Coordination Unit (Project Number: 2022-43341027-01).
Availability of data and materials
Data generated or analyzed during this study are provided in full within the published article.
Ethics approval and consent to participate
The experimental protocols comply with our institutional guidelines, which are equivalent to the “Guide for the Care and Use of Laboratory Animals (US National Institutes of Health, revised 1996)”. Approval was received from the Zonguldak Bulent Ecevit University Experimental Animal Ethics Committee (Protocol/Approval Number: 2021-30-02/12; Approval Date: December 02, 2021).
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc.) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

