Histopathological Characteristics according to the 2019 WHO Classification and VETC Pattern in Hepatocellular Carcinoma: A Retrospective Study in Viet Nam
- Department of Pathology, Hanoi Medical University. 01, Ton That Tung Street, Kim Lien Ward, Ha Noi City, Viet Nam
- Pathology and Cytology Center, Bach Mai Hospital. 78, Giai Phong Street, Kim Lien Ward, Ha Noi City, Viet Nam
- Department of Pathology, Phenikaa University. Nguyen Trac Street, Duong Noi Ward, Ha Noi City, Viet Nam
- Department of Pathology, Hospital of Hanoi Medical University. 01, Ton That Tung Street, Kim Lien Ward, Ha Noi City, Viet Nam
Abstract
Background: The histopathological heterogeneity of hepatocellular carcinoma (HCC) significantly influences patient management and prognosis. However, data on HCC subtypes classified by the 2019 World Health Organization (WHO) criteria and on the vessels-encapsulating tumor cluster (VETC) pattern—a recently described vascular architecture—remain scarce. This study aimed to delineate the histopathological subtypes of HCC according to the 2019 WHO classification, evaluate the prevalence of the VETC pattern, and investigate their clinicopathological associations. Methods: A retrospective, cross-sectional study was performed on 150 patients who underwent curative-intent hepatectomy for HCC at Bach Mai Hospital between 2019 and 2021. Results: The mean age was 56.3 ± 10.8 years, and the male-to-female ratio was 5.5:1. Chronic hepatitis B or C infection was present in 86 % of patients. Special histological subtypes—macrotrabecular and steatohepatitic (10.7 % each), scirrhous (8.7 %), clear cell (4.7 %), lymphocyte-rich (2.7 %), neutrophil-rich (0.7 %), and chromophobe (0.7 %)—were identified in 38.7 % of tumors. The microtrabecular architecture predominated (74 %), whereas glandular and macrotrabecular patterns were less frequent. The macrotrabecular pattern was significantly associated with elevated serum alpha-fetoprotein (AFP) levels and histological vascular invasion (p < 0.05). Similarly, the macrotrabecular and scirrhous subtypes correlated with high AFP levels and vascular invasion (p < 0.05). No significant association was found between histological subtypes and tumor stage. The VETC pattern was observed in 24 % of cases and was linked to vascular invasion (p < 0.05). Conclusion: This study underscores the broad spectrum of HCC subtypes and architectural patterns in Vietnamese patients and highlights the clinical relevance of the VETC phenotype. These insights may facilitate more refined prognostication and individualized therapeutic strategies.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy, and its incidence continues to rise 1. According to the GLOBOCAN 2022 statistics, HCC is the sixth most common cancer worldwide and the second most common in Viet Nam 2. HCC arises from a variety of aetiological factors, including viral infections (primarily hepatitis B and C), metabolic disorders such as obesity and diabetes, and chronic alcohol consumption 3. Each aetiology follows a distinct oncogenic pathway and may influence the histological characteristics of HCC. Histopathological examination remains essential for the definitive diagnosis of HCC, as it permits differentiation from cholangiocarcinoma, combined hepatocellular-cholangiocarcinoma, and metastatic liver neoplasms. It also enables assessment of histological subtype, differentiation grade, and vascular invasion. These parameters are pivotal for prognostication and therapeutic decision-making. HCC demonstrates marked histological heterogeneity, giving rise to distinct subtypes that correlate with specific clinical and biological features 4. Comprehensive insight into hepatocarcinogenesis and drug response therefore necessitates the evaluation of morphological heterogeneity, identification of predominant patterns, and correlation of phenotypes with molecular alterations of prognostic and therapeutic significance 5.
The World Health Organization (WHO) has successively updated the histopathological classification of liver tumours in 1976, 1990, 2000, 2010 and, most recently, 2019. The 2019 edition recognises additional histological subtypes, including scirrhous, fibrolamellar, clear-cell, steatohepatitic, macrotrabecular, lymphocyte-rich and neutrophil-rich variants 6. By contrast, the 2010 classification listed only the fibrolamellar, scirrhous, lymphoepithelioma-like and sarcomatoid variants. Beyond histological subtyping, the vessels-encapsulating-tumour-clusters (VETC) pattern, first delineated by Fang in 2015, has attracted interest owing to its prognostic relevance. Multiple studies have validated VETC as an independent prognostic factor in HCC 7. Accordingly, we conducted the present study to determine the distribution of histopathological subtypes according to the 2019 WHO classification, to assess the prevalence of the VETC pattern in HCC, and to explore their associations with selected clinical parameters.
Materials and Methods
Patients
This retrospective, descriptive, cross-sectional study analyzed HCC specimens resected between 2019 and 2021 at Bach Mai Hospital, Vietnam. Patients with mixed hepatocellular–cholangiocarcinoma were excluded. In total, 150 patients with HCC were included. Formalin-fixed, paraffin-embedded (FFPE) tissue samples were processed. Formalin-fixed tissue specimens were dehydrated through a graded ethanol series, cleared in xylene, and embedded in paraffin wax. Sections were cut from paraffin blocks at a thickness of 4 μm using a microtome 8. Hematoxylin-and-eosin (H&E)-stained slides from each resection specimen were reviewed. All slides were independently evaluated and scored by two pathologists.
Data collection
Clinical data included age, sex, hepatitis B virus (HBV) or hepatitis C virus (HCV) infection status, serum alpha-fetoprotein (AFP) concentration, Barcelona Clinic Liver Cancer (BCLC) stage, and maximum tumour diameter. Tumour grade (well, moderate, poor) and histological subtypes were classified according to the 2019 WHO Classification of Tumours of the Digestive System 6. The histological subtypes and patterns of HCC are described in
Histological subtypes và patterns of hepatocellular carcinoma
| Variables | Description |
|---|---|
| Histological subtype | |
| Not otherwise specified (NOS) | Also known as conventional HCC, diagnosed in the absence of specific histological subtypes described below. |
| Steatohepatitic | Tumour shows histological steatohepatitis |
| Clear cell | > 80% of tumour shows clear cell morphology |
| Macrotrabecular | Macrotrabecular growth pattern in > 50% of tumour |
| Scirrhous | > 50% of tumour shows dense intratumoural fibrosis |
| Fibrolamellar | Large eosinophilic tumour cells with prominent nucleoli, dense intratumoural fibrosis |
| Chromophobe | Light, almost clear cytoplasm (chromophobe) |
| Neutrophil-rich | Numerous and diffuse neutrophils within tumour |
| Lymphocyte-rich | Lymphocytes outnumber tumour cells in most fields |
| Growth pattern | |
| Microtrabecular | Presence of a microtrabecular pattern in at least 20% of the tumor |
| Glandular | Presence of a glandular pattern in at least 20% of the tumor |
| Solid | Presence of a solid pattern in at least 20% of the tumor |
| Macrotrabecular | Presence of a macrotrabecular pattern in at least 20% of the tumor |
| VETC | Presence of a VETC pattern in at least 5% of the tumor |
The occurrence and percentage of histomorphological growth patterns—macrotrabecular, microtrabecular, glandular and solid—were assessed. A pattern was considered present when it constituted ≥20 % of the tumour 10. Vessels-encapsulating tumour clusters (VETC) were recorded when they occupied ≥5 % of the H&E slide. VETC is characterised by sinusoid-like, endothelial-lined vessels that form a cobweb-like network completely surrounding cohesive tumour nests without intervening stroma 7. Vascular invasion was scored as present when tumour cells were located within a vascular lumen and attached to the vessel wall; when the vascular wall was unclear, immunohistochemistry for CD31 or CD34 was performed 12.
Statistical analysis
Data were analysed with SPSS version 26.0 (SPSS Inc., Chicago, IL, USA) 13. Quantitative variables are reported as means, and categorical variables as frequencies and percentages. Group comparisons were performed with Fisher’s exact test or Pearson’s χ² test, as appropriate. A two-sided P-value <0.05 was considered statistically significant 14.
Ethics approval
The study was approved by the Biomedical Research Ethics Committee of Hanoi Medical University (certificate No. 1052/GCN-HMUIRB).
Results
Clinical characteristics of patients with primary HCC
The mean age of the patients was 56.27 ± 11.9 years (range, 14–77 years). The highest prevalence was observed in the 60–69-year age group, accounting for 34.7%, whereas the <40-year group had the lowest proportion at 11.3%. Males represented 84.7% (n = 127) of the cohort, whereas females comprised 15.3% (n = 23), yielding a male-to-female ratio of 5.5:1. Elevated AFP concentrations (≥20 ng/mL) were detected in 48.7% of the patients. The principal etiology was chronic HBV or HCV infection, present in 86% of cases (126 HBV-related and 3 HCV-related). Most patients were diagnosed at Barcelona Clinic Liver Cancer (BCLC) stage A (74%), which is considered an early and potentially treatable stage. Regarding tumour size, 66% of patients had lesions measuring 2–5 cm, 28.7% had tumours >5 cm, and only 5.3% had tumours <2 cm. The proportions of well-, moderately-, and **poorly-**differentiated grades were 16%, 72.7%, and 11.3%, respectively. Vascular invasion was documented in 48 cases (32%). The clinicopathological characteristics are summarised in
Summary of the clinicopathological characteristics of primary hepatocellular carcinoma patients
| Variables | Characteristics | Number 150 | Percentage (%) |
|---|---|---|---|
| Age | <40 | 17 | 11.3 |
| 40-59 | 22 | 14.7 | |
| 50-59 | 41 | 27.3 | |
| 60-69 | 52 | 34.7 | |
| ≥70 | 18 | 12.0 | |
| Sex | Male | 127 | 84.7 |
| Female | 23 | 15.3 | |
| AFP level | <20 ng/ml | 77 | 51.3 |
| ≥20 ng/ml | 73 | 48.7 | |
| Etiology | HBV/HCV | 129 | 86.0 |
| Alcohol | 8 | 5.3 | |
| Unknown | 13 | 8.7 | |
| BCLC Stage | 0 | 6 | 4.0 |
| A | 111 | 74.0 | |
| B | 21 | 14.0 | |
| C | 12 | 8.0 | |
| Tumor Size | <2 cm | 8 | 5.3 |
| 2–5 cm | 99 | 66.0 | |
| >5 cm | 43 | 28.7 | |
| Tumor grade | Well | 24 | 16.0 |
| Moderately | 109 | 72.7 | |
| Poorly | 17 | 11.3 | |
| Vascular invasion | 48 | 32.0 | |
Histopathological subtypes and correlation
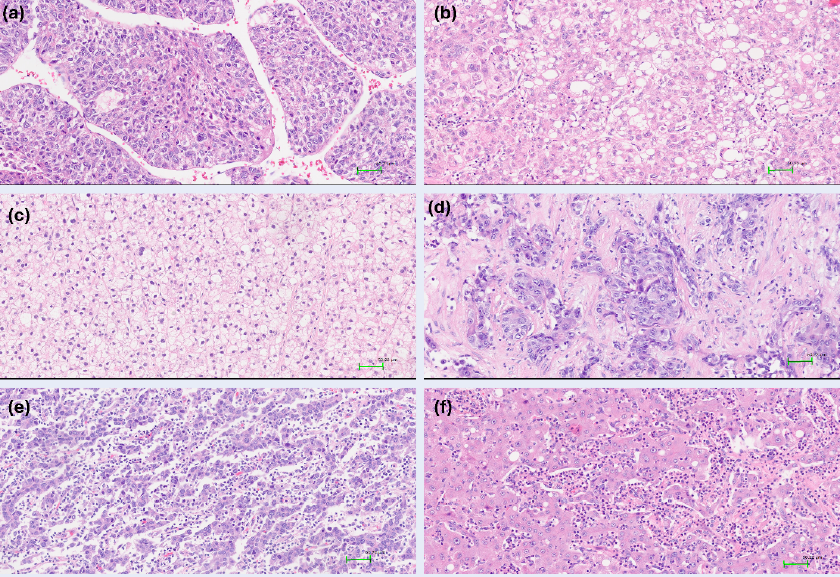
Distinct histological subtypes accounted for 38.7% of cases. According to the WHO classification, the following HCC subtypes were identified: steatohepatitic (10.7%), macrotrabecular (10.7%), scirrhous (8.7%), clear cell (4.7%), and rarer variants such as lymphocyte-rich (2.7%), chromophobe (0.7%), and neutrophil-rich (0.7%). An illustration is provided in Figure 1. A correlation was observed between histological subtype and serum AFP level: high AFP (>20 ng/mL) occurred significantly more often in the macrotrabecular, scirrhous, and steatohepatitic subtypes (p < 0.05). The macrotrabecular, scirrhous, and clear-cell subtypes also exhibited higher rates of vascular invasion (p < 0.05). No significant association was found between subtype and disease stage (p = 0.85) (
Relationships of histological subtypes with some clinicopathological features of patients
| Subtypes | Count (Freq) | AFP (≥20 ng/ml) | BCLC stage | Vascular invasion | |
|---|---|---|---|---|---|
| 0/A | B/C | ||||
| NOS | 92 (61.3%) | 37 (40.2%) | 72 (78.3%) | 20 (21.7%) | 26 (28.3%) |
| Macrotrabecular | 16 (10.7%) | 10 (62.5%) | 11 (68.8%) | 5 (31.3%) | 10 (62.5%) |
| Steatohepatitic | 16 (10.7%) | 9 (56.3%) | 14 (87.5%) | 2 (12.5%) | 3 (18.8%) |
| Scirrhous | 13 (8.7%) | 11 (84.6%) | 10 (76.9%) | 3 (23.1%) | 6 (46.2%) |
| Clear cell | 7 (4.7%) | 3 (42.9%) | 5 (71.4%) | 2 (28.6%) | 3 (42.9%) |
| Other | 6 (4.0%) | 3 (50%) | 5 (83.3%) | 1 (16.7%) | 0 (0%) |
Subtypes of hepatocellular carcinoma (HE stain,×200). a. Macrotrabecular subtype:the tumor composed of trabeculae with >10 cells thickness. b. Steatohepatitic subtype: the tumor cells contain cytoplasmic fat vacuoles. c. Clear cell subtype: the tumor cells have abundant clear cytoplasm. d. Scirrhous subtype: thick and dense fibrous collagen is traversing irregular tumor cluster. e. Lymphocyte-rich subtype: tumor-infiltrating lymphocytes outnumber tumor cells. f. Neutrophil-rich subtype: the tumor is characterized by marked neutrophilic infiltration. Abbreviation: H&E, hematoxylin and eosin
Histopathological patterns and correlation
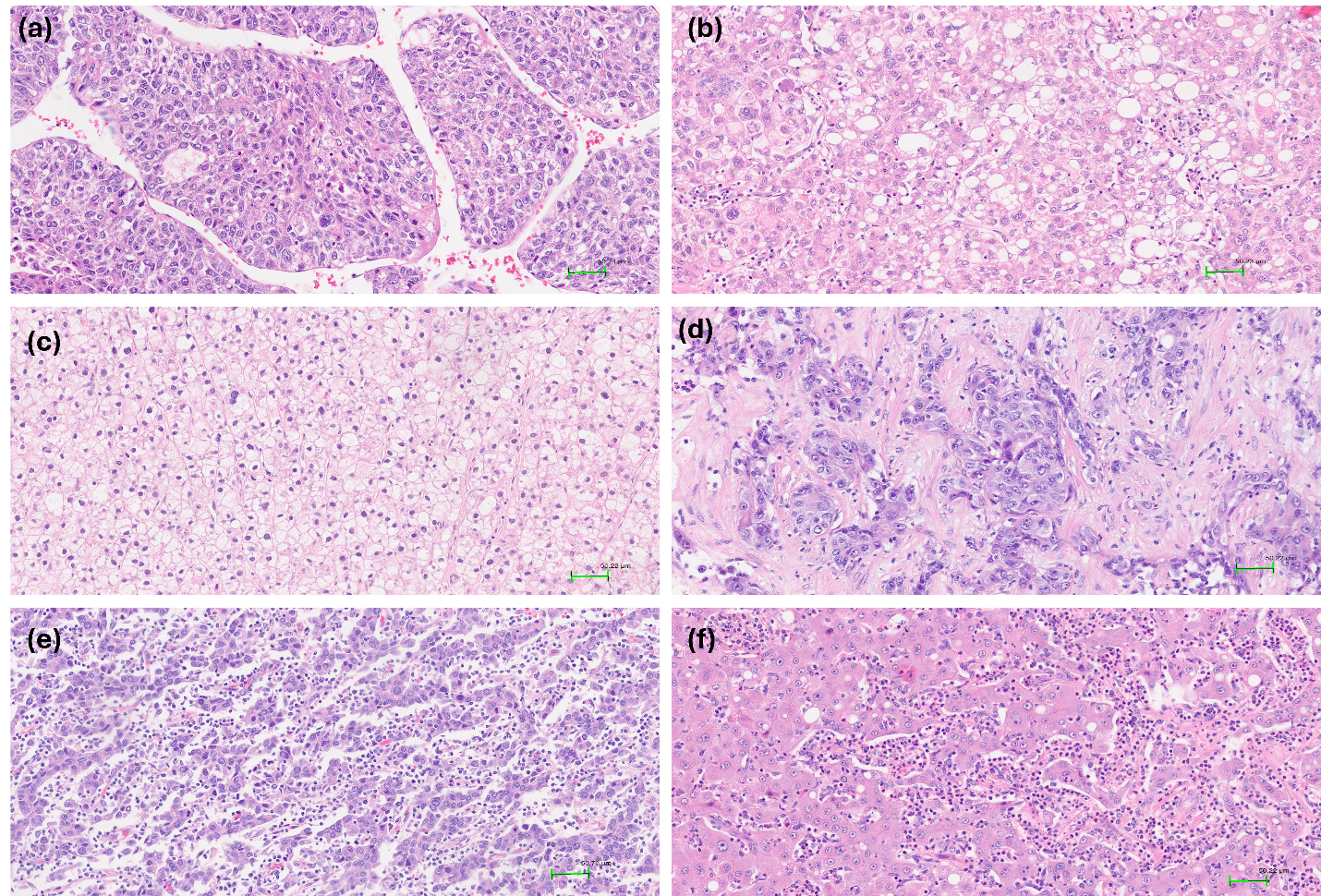
Among growth patterns, the microtrabecular pattern was most prevalent, observed in 74% of cases. The solid, glandular, and macrotrabecular patterns were present in 27.3%, 14%, and 19.3% of tumours, respectively. An illustration is shown in Figure 2. Notably, classification of the macrotrabecular subtype requires the pattern to occupy >50% of the lesion, whereas the macrotrabecular pattern is recorded when it comprises >20% of the tumour area; this distinction may explain the differing prevalence reported here. No significant associations were detected between microtrabecular, solid, or glandular patterns and serum AFP, stage, or vascular invasion. In contrast, the macrotrabecular pattern was significantly associated with elevated AFP (≥20 ng/mL) and a higher frequency of vascular invasion than tumours lacking this pattern (p < 0.05). The VETC pattern was identified in 36 cases (24%). Vascular invasion occurred in 50% of VETC-positive tumours, whereas its incidence was markedly lower in VETC-negative tumours (p < 0.05) (
Relationships of histological patterns with some clinicopathological features of patients
| Count (Freq) | AFP (≥20 ng/ml) | BCLC stage | Vascular invasion | |||||
|---|---|---|---|---|---|---|---|---|
| 0/A | B/C | |||||||
| Microtrabecular | 111 (74%) | 55 (49.5%) | 0.715 | 89 (80.2%) | 22 (19.8%) | 0.277 | 33 (29.7%) | 0.315 |
| Solid | 41 (27.3%) | 24 (58.5%) | 0.138 | 32 (78%) | 9 (22%) | 0.993 | 16 (39%) | 0.258 |
| Glandular | 21 (14%) | 8 (38.1%) | 0.296 | 17 (81%) | 4 (19%) | 0.725 | 8 (38.1%) | 0.518 |
| Macrotrabecular | 29 (19.3%) | 19 (65.5%) | 0.043* | 20 (69%) | 9 (31%) | 0.191 | 16 (55.2%) | 0.003* |
| VETC | 36 (24%) | 18 (50%) | 0.854 | 27 (75%) | 9 (25%) | 0.618 | 18 (50%) | 0.008* |
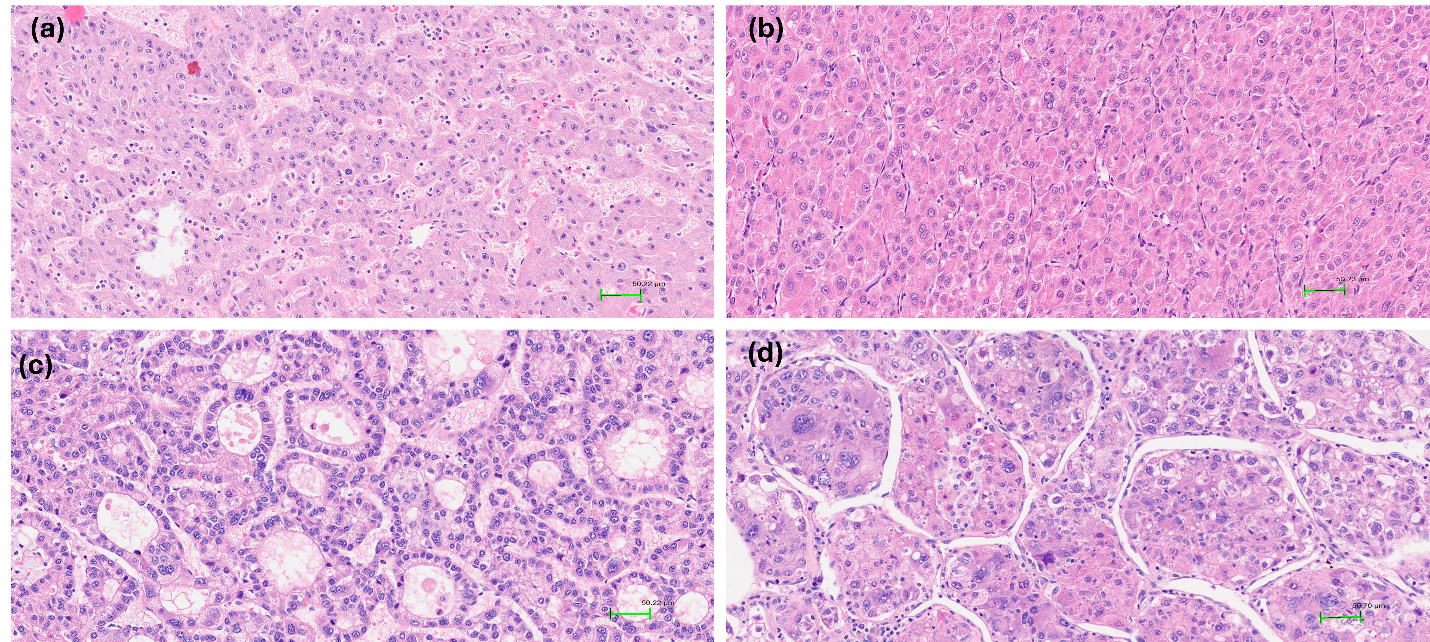
Histological patterns in hepatocellular carcinoma (HE stain, x200).a. The tumor cells grow in microtrabecular pattern, 2–4 cells thick. b. The tumor cells grow in a solid pattern without trabecular or pseudoglandular growth. c. The tumor cells grow in a pseudoglandular pattern with glandular or acinar structure. d. Macrotrabecular pattern with thick trabeculae more than 10 cell layers.
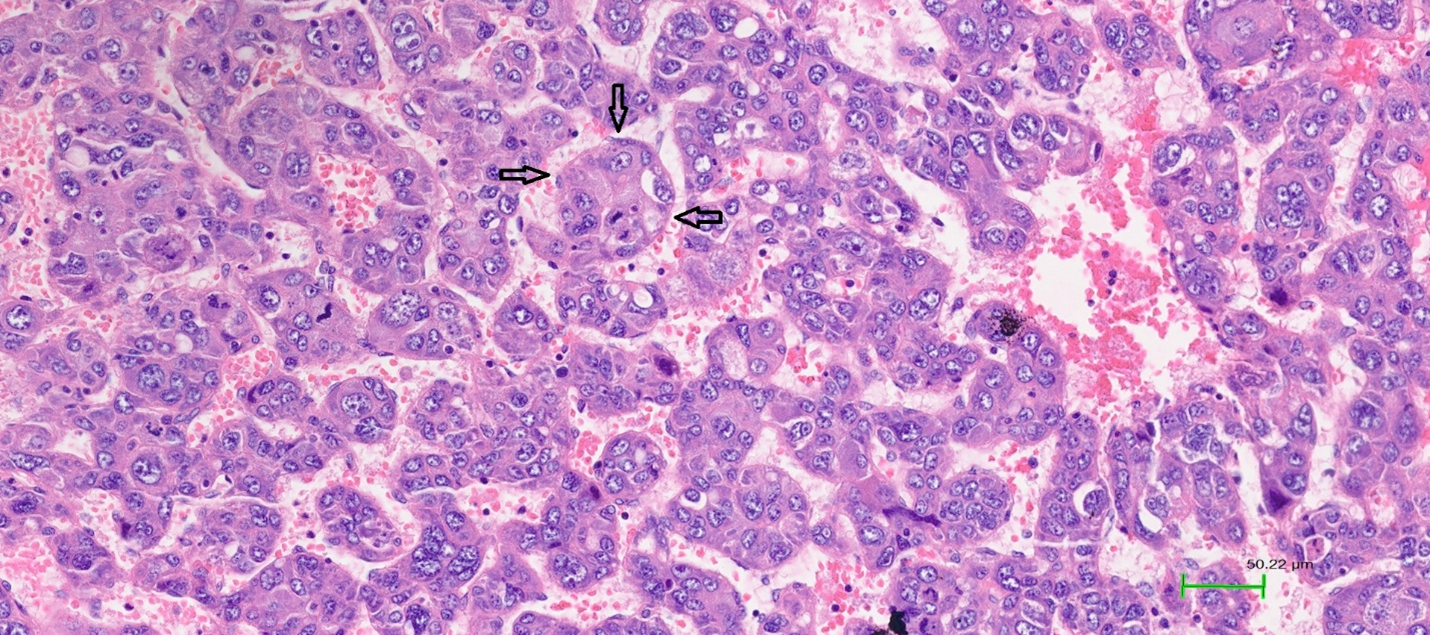
VETC pattern in hepatocellular carcinoma (HE stain, ×200). Tumor cells are encapsulated by blood vessels arranged in a network pattern. The arrow indicates the endothelial layer of blood vessels surrounding the tumor cell cluster.
Discussion
Hepatocellular carcinoma (HCC) predominantly affects men and individuals aged 50–70 years. Our findings mirror those of several global studies, revealing a male-to-female ratio of 5.5 : 1 and a mean patient age of 56.27 years. Chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection is the principal cause of HCC, although some European studies implicate alcohol misuse as the leading aetiology 10. In Viet Nam, HBV and HCV infection remain the primary aetiological factors because of their high prevalence (10–15 % of the population) and suboptimal control measures. Conversely, worldwide epidemiology is shifting toward metabolic-associated fatty liver disease, largely attributable to effective HBV vaccination programmes and widespread HBV/HCV screening and treatment. These observations underscore the urgent requirement to broaden HBV vaccination coverage, intensify viral-hepatitis screening, and institute early antiviral therapy to mitigate the HCC burden in Viet Nam 15,16.
In the present study, we characterised the morphological diversity and heterogeneity of HCC. Of the 150 cases analysed, 61.3 % were conventional HCC. The special histological subtypes comprised the macrotrabecular (10.7 %), steatohepatitic (8.7 %) and scirrhous (8.7 %) variants. Rare subtypes, including lymphocyte-rich, neutrophil-rich and chromophobe-cell tumours, were also encountered. Beyond these specific subtypes, we evaluated architectural patterns. Among the four patterns—microtrabecular, solid, glandular and macrotrabecular—the microtrabecular pattern was most prevalent, and approximately one-third of tumours displayed mixed architectures. Emerging evidence indicates that morphology may reflect underlying molecular alterations; hence, correlating morphological features with tumour molecular biology and incorporating this information into pathology reports could inform targeted therapy.
The macrotrabecular subtype was introduced as a novel histological category in the 2019 WHO classification. Edmondson and Steiner originally described trabecular HCC as fragmented and discrete versus thick and continuous, the latter approximating the macrotrabecular structure recognised by the 2019 WHO classification 17. Calderaro et al. proposed a ≥50 % threshold for macrotrabecular designation, subsequently endorsed by the WHO. Ziol et al. documented a macrotrabecular rate of 12 % in 237 resection and 284 biopsy specimens, whereas Rastogi et al. reported 9.2 % in 217 cases 9,11,18. In our cohort, the macrotrabecular subtype accounted for 10.7 % of tumours. This subtype is frequently associated with TP53 mutations and/or fibroblast growth factor-19 (FGF19) amplification. Angiogenesis characterised by angiopoietin-2 and vascular endothelial growth factor-A (VEGF-A) co-expression is a hallmark of macrotrabecular HCC. Multiple studies link this subtype to elevated serum α-fetoprotein (AFP), vascular invasion, early recurrence and poor prognosis 9,19. Consistent with these data, the macrotrabecular subtype in our series correlated with higher AFP concentrations (> 20 ng mL⁻¹) and vascular invasion; however, its distribution across tumour stages was not statistically significant.
The steatohepatitic subtype, first described in 2010, is molecularly characterised by activation of the interleukin-6/Janus kinase/signal transducer and activator of transcription (IL-6/JAK/STAT) pathway. In our series, 75 % of steatohepatitic HCCs occurred in HBV-positive patients, whereas 25 % had undetermined aetiology. Reported prevalence varies from 6 % to 10 % across studies, and prognostic implications remain inconsistent 6,20. In our cohort, the steatohepatitic subtype was associated with moderate AFP levels and a lower incidence of vascular invasion compared with the macrotrabecular and scirrhous subtypes.
The clear-cell subtype is uncommon and exhibits cytoplasmic clearing secondary to glycogen accumulation. Isocitrate dehydrogenase-1 (IDH1) mutations, present in 3–6 % of HCCs, have been identified in this variant. Clear-cell HCC generally confers a more favourable prognosis than conventional HCC. We identified seven cases; one-third were stage B/C and three displayed vascular invasion. Prognosis deteriorates when clear-cell morphology coexists with other subtypes, and transformation to conventional HCC with increasing malignancy has been documented 21.
The scirrhous subtype constituted 8.7 % of our cases and was characterised by elevated AFP (> 20 ng mL⁻¹) and frequent vascular invasion; all patients were HBV-positive. Outcomes are poor, and overall survival is inferior to that observed in unselected HCC 22.
With respect to architectural patterns, the microtrabecular pattern predominated (74 %), whereas the solid, glandular and macrotrabecular patterns were less common. The macrotrabecular pattern correlated with higher AFP levels and increased vascular invasion. Rastogi et al. similarly noted an association between the macrotrabecular pattern and high AFP, advanced stage, poor differentiation and larger tumours 11. When macrotrabecular architecture comprises > 50 % of tumour parenchyma, the lesion is categorised as the macrotrabecular subtype, as discussed above.
Vessels-encapsulating tumour clusters (VETC) represent a recently recognised prognostic vascular pattern, defined by sinusoidal vessels enveloping cohesive tumour islands in a spider-web configuration. Although Sugino described comparable vasculature in 2004, the term VETC was first employed by Fang in 2015. Subsequent studies have confirmed VETC as an independent adverse prognostic factor that influences therapeutic decision-making. VETC frequently co-occurs with large tumours, vascular invasion, hepatic and pulmonary metastases and high AFP concentrations 23. Huang et al. reported recurrence rates of 66 % in VETC-positive versus 47.9 % in VETC-negative patients 24. Using a 5 % cutoff, we observed VETC in 24 % of tumours. Reported frequencies include 39 % in Renne’s cohort (n = 541) and 23.8 % in Akiba’s series (n = 985); both studies associated VETC with reduced survival and increased recurrence 7. Consistent with these findings, our data revealed a high incidence of vascular invasion in VETC-positive cases. Mechanistically, VETC is closely linked to β-catenin activation and glutamine synthetase expression, facilitating collective tumour dissemination without endothelial breach. VETC also correlates with increased VEGF-A expression, potentially enhancing responsiveness to VEGF inhibitors such as sorafenib and bevacizumab 23.
This investigation is among the first in Viet Nam to apply the 2019 WHO histopathological classification and VETC assessment to HCC. Limitations include the absence of overall and disease-free survival data, a relatively small sample size, and the lack of molecular characterisation. Future studies incorporating larger cohorts, long-term follow-up, and comprehensive immunohistochemical and molecular profiling are warranted to clarify the prognostic significance of histological subtypes and architectural patterns, particularly in Asian populations.
Conclusion
This study characterizes the spectrum of histological subtypes and growth patterns observed in Vietnamese patients with hepatocellular carcinoma (HCC). In addition to the macrotrabecular, steatohepatitic and scirrhous subtypes, several uncommon variants were detected, including the neutrophil-rich and lymphocyte-rich forms defined in the 2019 WHO classification. The macrotrabecular and scirrhous patterns were significantly associated with increased serum alpha-fetoprotein (AFP) concentrations and vascular invasion, whereas the VETC architecture was linked to a greater frequency of vascular invasion. These findings broaden clinicians’ understanding of HCC pathology and may inform patient risk stratification and therapeutic planning. The principal limitation of this study is the absence of survival data; consequently, prospective analyses incorporating larger cohorts and extended follow-up are warranted to determine the prognostic significance of the identified histological subtypes and architectural patterns.
Abbreviations
AFP: Alpha-fetoprotein; BCLC: Barcelona Clinic Liver Cancer; FFPE: Formalin-fixed, paraffin-embedded; FGF19: Fibroblast growth factor-19; H&E: Hematoxylin and eosin; HCC: Hepatocellular carcinoma; HBV: Hepatitis B virus; HCV: Hepatitis C virus; IDH1: Isocitrate dehydrogenase-1; IL-6/JAK/STAT: Interleukin-6/Janus kinase/Signal transducer and activator of transcription; NOS: Not otherwise specified; VEGF-A: Vascular endothelial growth factor-A; VETC: Vessels-encapsulating tumor clusters; WHO: World Health Organization Molecular Data: Acknowledge the lack of molecular profiling as a limitation in this study.
Acknowledgments
We would like to thank Hanoi Medical University and Bach Mai Hospital for their approval.
Author contributions
DMK, NVH: conceptualization, methodology, writing—original draft preparation; TNM: visualization, methodology, software, data curation; NVH: validation, investigation, supervision. All the authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc) and AI-assisted technologies in the writing process before submission.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Biomedical Research of Hanoi Medical University under certificate number 1052/GCN-HMUIRB. All participants provided written informed consent
Competing interests
The authors declare that they have no competing interests.

