Unraveling the relationship between thyroid peroxidase antibodies and thyroid hormones: A partial correlation approach in autoimmune thyroid disease
- Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Malaya, 50603 Kuala Lumpur, Malaysia
- Branch of Clinical Science, College of Medicine, University of Sulaimani, Sulaymaniyah, Kurdistan region, Iraq
- Anwar Sheikha Medical City, Qaiwan Health, Sulaymaniyah, Kurdistan Region, Iraq
- Pharmacy Department, College of Medicine, Komar University of Science and Technology, Sulaimani, Iraq
- Department of Medicine, College of Veterinary Medicine, University of Sulaimani, Sulaimani, Iraq
- Department of Internal Medicine, College of Medicine, University of Sulaimani, Sulaimani, Iraq
- Department of Biochemistry, College of Medicine, University of Karbela, Karbela, Iraq
Abstract
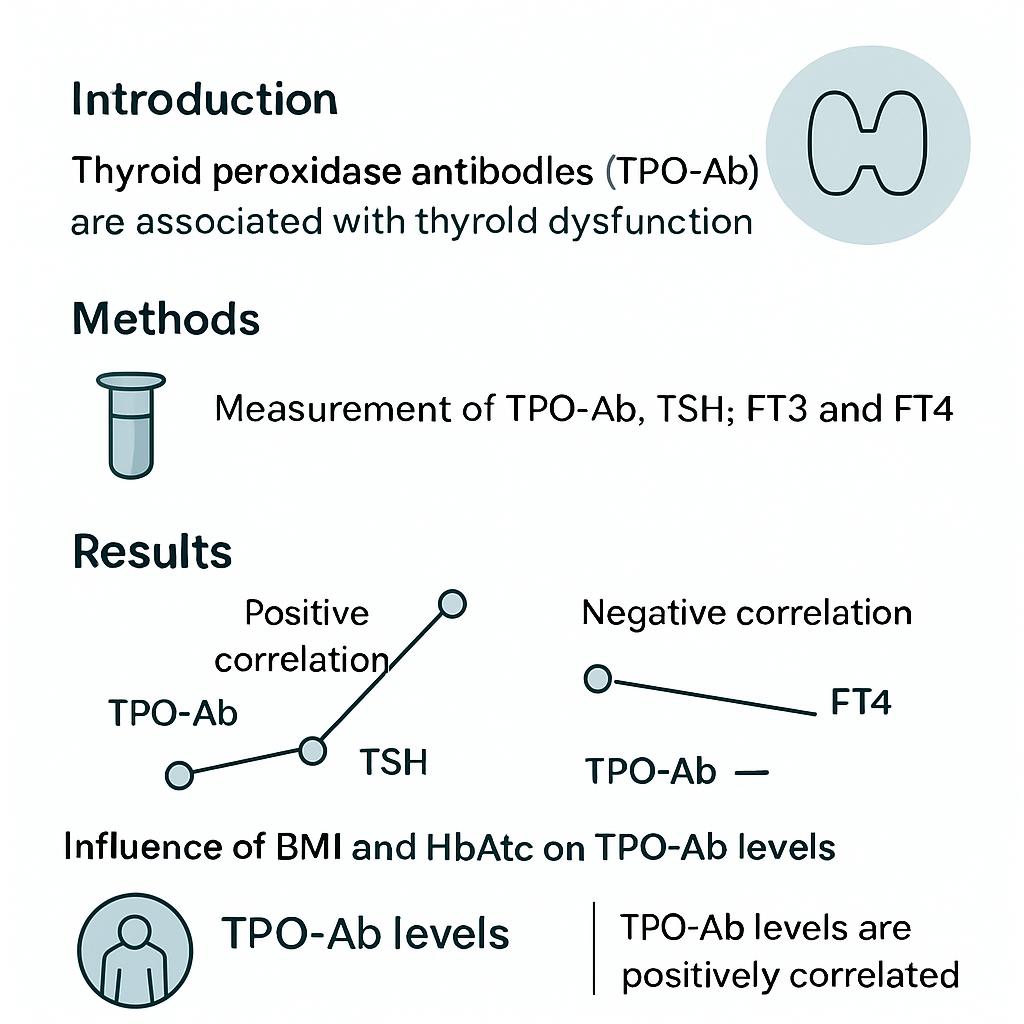
Introduction: Autoimmune thyroid disease (AITD) is a T-cell mediated disorder characterized by the presence of thyroid autoantibodies, particularly thyroid peroxidase antibodies (TPO-Ab), which serve as specific biomarkers of thyroid autoimmunity. The relationship between TPO-Ab and thyroid hormones is influenced by several confounding factors, potentially leading to inconsistent findings across studies.
Methods: This cross-sectional study investigated 47 patients with AITD (35 women, 12 men; mean age 40 years) receiving levothyroxine (LT4) therapy, all with serum TPO-Ab ≥16 IU/mL. Anthropometric, glycemic, and lipid indices were measured alongside thyroid function tests, and correlations were assessed using zero-order and partial correlation analyses.
Results: A significant positive correlation was found between TPO-Ab and thyroid-stimulating hormone (TSH) (r = 0.510, p < 0.001). Adjusting for age, body mass index (BMI), fasting glucose, HbA1c, and LDL-c reduced this correlation, while controlling for triglycerides (TG) and HDL-c increased it. Similar trends were observed between TPO-Ab and free thyroid hormones. Multivariate regression showed that 29.1 % of TPO-Ab variability was explained by the tested covariates, highlighting the interplay between metabolic and immunological factors. The influence of lipid parameters suggests that lipid metabolism may directly modulate autoimmunity and thyroid function. Moreover, the use of exogenous LT4, known to lower TG and HDL-c, further complicates the interpretation of antibody-hormone interactions.
Conclusion: In conclusion, applying partial correlation analysis provides a more accurate assessment of the link between TPO-Ab and thyroid hormones compared to simple correlations, and supports the need for controlling metabolic and demographic covariates in both clinical practice and research to avoid misleading associations in autoimmune thyroid disease.
INTRODUCTION
Autoimmune thyroid diseases (AITD), also referred to as autoimmune thyroiditis, are organ-specific, T-cell-mediated disorders characterized by immune dysregulation and the production of thyroid autoantibodies, particularly thyroid peroxidase antibodies (TPO-Ab) and thyroglobulin antibodies (TG-Ab), which are widely recognized biomarkers of thyroid autoimmunity 1. Several factors influence TPO-Ab concentrations and complicate their clinical interpretation. For instance, non-modifiable determinants such as age and female sex are associated with higher antibody titers even in the absence of overt thyroid dysfunction 2. Obesity has likewise emerged as an important risk factor, with studies reporting significantly higher TPO-Ab levels among obese individuals 3. Conversely, weight loss in patients with Hashimoto’s thyroiditis has been shown to reduce antibody titers and improve thyroid hormone profiles, indicating that body weight is an important determinant of thyroid function 4.
Lifestyle and metabolic factors can modulate thyroid autoimmunity. Cigarette smoking has been linked to decreased serum TPO-Ab and thyroid-stimulating hormone (TSH) concentrations, whereas dyslipidemia is common in subclinical hypothyroidism and often correlates with antibody positivity 5,6. Elevated total cholesterol, triglyceride, and low-density lipoprotein cholesterol (LDL-C) levels are particularly prevalent among women with autoimmune thyroid disease 7. Conversely, lower fasting glucose concentrations are typically associated with negative TPO-Ab status in euthyroid individuals 8.
Previous studies have reported variable associations between TPO-Ab and thyroid function tests. Whereas some investigations identified strong positive correlations with triiodothyronine (T3), thyroxine (T4), and TSH, others revealed nonlinear or inconsistent relationships after adjustment for age, sex, or smoking status 9,10. These discrepancies emphasize the necessity of employing analytical methods that adequately control for confounding, because simple correlations may yield biased or misleading conclusions.
The present study investigated the association between thyroid peroxidase antibodies (TPO-Ab) and thyroid-hormone parameters in patients with autoimmune thyroid disease receiving levothyroxine (LT4) replacement. Zero-order and partial-correlation analyses were conducted. Anthropometric, glycaemic, and lipid indices were entered as covariates to evaluate their potential confounding influence on the TPO-Ab–TSH relationship. Accounting for these variables is expected to improve understanding of antibody–hormone interactions and to provide a more robust framework for interpreting TPO-Ab levels in clinical and research contexts.
MATERIALS AND METHODS
Study setting
This cross-sectional, observational study was conducted at the College of Pharmacy, University of Sulaimani, in collaboration with High Quality Hospital/Anwar Sheikha Medical City (AMC), Sulaimani, Iraq. Patient recruitment and data collection were conducted between November 2024 and June 2025. The study enrolled adult patients who were receiving LT4 therapy for autoimmune thyroiditis and had recent biochemical confirmation of elevated TPO-Ab levels.
Ethical Consideration
This study was approved by the Ethics Committee of the College of Pharmacy, University of Sulaimani, Iraq (Approval No. PH65-22). All procedures were carried out in strict accordance with the ethical standards of the Declaration of Helsinki 11 for research involving human participants. Written informed consent was obtained from every participant before enrollment, and the confidentiality of all patient data was rigorously protected throughout the study and after its completion.
Patients Selection
A total of 47 patients with autoimmune thyroid disease (AITD) were recruited; the cohort comprised 35 females and 12 males, with a mean age of 40 years. The diagnosis of AITD was confirmed by an endocrinologist on the basis of clinical evaluation and serological evidence of elevated thyroid peroxidase antibody (TPO-Ab) titers (≥16 IU/mL). Ultrasound findings were not required for inclusion, as the study primarily focused on biochemical and immunological correlations. All participants were receiving stable doses of levothyroxine (LT4) therapy for a minimum of six months prior to inclusion. LT4 doses were titrated to maintain serum thyroid-stimulating hormone (TSH) concentrations within the therapeutic range of 0.5–4.5 µIU/mL, according to endocrinology follow-up records. No participants were newly initiated on LT4 or undergoing dose adjustment during the data-collection period. All participants had serum TPO-Ab levels ≥16 IU/mL. Patients were consecutively enrolled from endocrinology and internal medicine outpatient clinics at AMC. Inclusion criteria comprised adults of both sexes with confirmed AITD and ongoing LT4 replacement therapy. All participants were nonsmokers at enrolment. Female participants were premenopausal or perimenopausal; postmenopausal women were excluded to minimize hormonal variability. Exclusion criteria included thyroidectomy; the presence of other autoimmune or systemic inflammatory diseases; malignancies of the thyroid gland; regular alcohol intake; pregnancy; and the use of medications such as proton-pump inhibitors, corticosteroids, supplements (e.g., calcium or iron), and lipid-lowering agents that could interfere with thyroid function or antibody levels. A detailed medical history and demographic profile were obtained from each patient, followed by a physical examination. Anthropometric data, including body weight and height, were recorded, and body mass index (BMI) was calculated.
Sample Size Determination
The sample size calculations were performed with GPower version 3.1. For the planned correlation analyses, the significance level was set at α = 0.05 and the statistical power at 1 – β = 0.95. An anticipated correlation coefficient of ρ = 0.50 was adopted on the basis of previous reports describing moderate-to-strong relationships between TPO-Ab concentrations and TSH levels in cohorts with autoimmune thyroid disease (AITD) (Shimizu et al., 2023; Al-Rabia, 2017). A sensitivity analysis was also carried out in GPower 3.1 (test family: “Exact”; statistical test: “Correlation: Bivariate normal model”; two-tailed; α = 0.05; power = 0.95; n = 47). This analysis indicated a minimum detectable effect size of ρ = 0.49, confirming that the current sample is adequately powered to identify moderate-to-large correlations between the variables of interest. For the Pearson two-tailed correlation, the required sample size was additionally confirmed with the Fisher z-transformation formula:
Where: n = required sample size, ρ = expected correlation (effect size), α = significance level (e.g., 0.05), and β = Type II error rate (1-power) With α = 0.05 (two-tailed), power = 0.95, and expected correlation ρ = 0.50: n ≈ 46 → Recruiting 47 participants met this requirement.
Laboratory investigations
Venous blood samples were collected from each participant after at least 12 h of overnight fasting. Approximately 10 mL of blood were drawn from the antecubital vein, placed into plain tubes, and promptly delivered to the AMC central laboratory for processing. Routine biochemical analyses comprised fasting serum glucose (FSG), glycated hemoglobin (HbA1c), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-c), and high-density lipoprotein cholesterol (HDL-c). The triglyceride-glucose (TyG) index, a validated surrogate of insulin resistance, was computed according to the following equation:
Serum levels of TSH, FT3, FT4, and TPO-Ab were quantified using an automated chemiluminescent immunoassay (CLIA) system (Architect i2000SR; Abbott Diagnostics, USA). All assays were performed in accordance with the manufacturer’s instructions. The analytical performance of the system was verified before sample analysis, with intra-assay and inter-assay coefficients of variation (CV) maintained below 5 % and below 8 %, respectively. Fasting serum glucose (FSG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured enzymatically using standard colorimetric assays on an automated chemistry analyzer (AU5800; Beckman Coulter, USA). HbA1c was determined by ion-exchange high-performance liquid chromatography (HPLC).
Statistical Analysis
Data were analyzed with the Statistical Package for the Social Sciences (SPSS), version 24.0 (IBM Corp., Chicago, IL, USA). Continuous variables are presented as means ± standard deviations (SDs), whereas categorical variables are described as frequencies and percentages. Independent two-sample t-tests were employed to compare clinical and biochemical parameters between male and female participants. Associations between TPO-Ab and thyroid hormones (TSH, FT3, FT4) were initially explored using Pearson correlation coefficients. To adjust for potential confounders, partial correlation analyses were conducted with adjustment for demographic (age), anthropometric (BMI), glycemic (FSG, HbA1c), and lipid indices (TC, TG, LDL-c, HDL-c, TyG index). Ninety-five percent confidence intervals (95 % CI) for each correlation coefficient (r) were calculated using Fisher z-transformation.
Multivariable linear regression was performed to identify independent determinants of serum TPO-Ab concentrations, with age, body mass index (BMI), glycaemic parameters, and lipid-profile indices entered as covariates. The model yielded standardized β-coefficients, the coefficient of determination (R²), and the overall F-statistic. Statistical significance was defined as a two-tailed p-value ≤ 0.05. To limit type I error arising from multiple comparisons, p-values obtained from the partial correlation analyses were corrected by the Benjamini–Hochberg false-discovery-rate (FDR) procedure. The distribution of all continuous variables was assessed with the Shapiro–Wilk test as well as visual inspection of histograms and Q–Q plots. Although several variables exhibited minor departures from normality, parametric tests were retained because the sample size was > 30 and visual inspection suggested an approximately normal distribution.
RESULTS
Baseline Characteristics
A total of 47 patients with autoimmune thyroid disease (AITD) were enrolled, including 35 women (74.5 %) and 12 men (25.5 %). The cohort’s mean age was 40.0 ± 13.2 years. Mean body weight and body-mass index (BMI) were 77.1 ± 14.6 kg and 27.0 ± 5.2 kg/m², respectively. Sex-specific analysis showed that women exhibited a significantly greater BMI than men (27.8 ± 5.6 vs 24.9 ± 2.9 kg/m²; p = 0.027). Conversely, men displayed higher mean high-density lipoprotein cholesterol (HDL-c) concentrations than women (62.5 ± 17.0 vs 49.2 ± 10.8 mg/dL; p = 0.023). No sex-related differences were detected in thyroid-function tests (TSH, FT3, FT4, TPO-Ab) or glycaemic parameters.
Characteristics of the study’s participants
| Variables | Females (n=35) | Males (n=12) | Total (n=47) | |
|---|---|---|---|---|
|
Age (year) Body weight (kg) Body mass index (kg/m2) Thyroid stimulating hormone (µU/mL) Free triiodothyronine hormone (pmol/L) Free thyroxine hormone (pmol/L) Thyroid peroxidase antibodies (IU/mL) Fasting serum glucose (mg/dL) Glycosylated hemoglobin (%) Fasting lipid profile (mg/dL) TC (mg/dL) TG (mg/dL) HDL-c (mg/dL) LDL-c (mg/dL) Triglyceride-glucose index |
41.1±13.3 76.1±15.6 27.8±5.6 6.68±1.95 3.58±1.36 15.0±2.5 39.17±16.76 183.7±75.4 5.52±1.1 237.8±57.5 173.5±66.0 49.2±10.8 156.8±50.7 7.29±2.09 |
36.7±13.1 79.8±11.3 24.9±2.9 6.17±1.33 3.42±1.46 15.5±2.2 34.57±15.31 214.4±76.0 5.19±1.1 256.0±57.5 203.5±91.9 62.5±17.0 168.7±36.0 7.18±2.44 |
40.0±13.2 77.1±14.6 27.0±5.2 6.55±1.81 3.54±1.37 15.14±2.44 37.98±16.37 191.6±75.9 5.44±1.09 242.4±61.8 181.1±73.6 52.6±13.8 159.8±47.3 7.26±2.16 |
0.328 0.387 0.027 0.315 0.750 0.539 0.392 0.242 0.363 0.450 0.314 0.023 0.383 0.891 |
Potential Association between TPO-Ab and Thyroid Hormones
Analysis of the entire cohort revealed a significant positive correlation between TPO-Ab and TSH (r = 0.510, df = 45, p < 0.001). This association persisted in both sexes and remained evident after adjustment for individual covariates, although its magnitude varied. Specifically, adjustment for age, BMI, fasting serum glucose, HbA1c, and LDL-c resulted in a slight decrease in the correlation coefficient, whereas adjustment for TG and HDL-c produced a marginal increase (r rose from 0.510 to 0.522).
The correlation between TPO-Ab and FT3 was negative (r = –0.553, p < 0.001). After covariate adjustment, the same pattern was observed: the correlation weakened after controlling for age, BMI, and LDL-c, but strengthened modestly after controlling for TG, HDL-c, and the TyG index. Likewise, TPO-Ab showed a negative association with FT4 (r = –0.406, p = 0.005). As with FT3, this association displayed only minor variations after adjustment for age, BMI, and glycaemic indices, whereas inclusion of TG, HDL-c, and the TyG index slightly amplified the association. Following false-discovery-rate correction using the Benjamini–Hochberg method, all correlations between TPO-Ab and thyroid hormones remained statistically significant. For the TPO-Ab–TSH and TPO-Ab–FT3 pairs, the adjusted q-values were ≤ 0.01, whereas for the TPO-Ab–FT4 pair the q-values ranged from 0.0077 to 0.0080. The complete zero-order and partial correlation results are summarised in
Impact of lipid and glycemic indices on the relationship between thyroid peroxidase antibodies with thyroid stimulating hormone by using zero-order (or none) and partial correlations.
| Controllers | Zero-order correlation coefficient | Partial correlation coefficient | Changes in correlation coefficient | r ( | Adjusted | 95 % CI for r |
|---|---|---|---|---|---|---|
| Age | 0.510 (<0.001) | 0.496 (<0.001) | ↓ | 0.496 (<0.001) | 0.001 | 0.24 – 0.69 |
| BMI | 0.510 (<0.001) | 0.476 (0.001) | ↓ | 0.476 (0.001) | 0.001 | 0.22 – 0.67 |
| Glycemic indices | ||||||
| FSG | 0.510 (<0.001) | 0.501 (<0.001) | ↓ | 0.510 (<0.001) | 0.001 | 0.26 – 0.70 |
| HbA1c (%) | 0.510 (<0.001) | 0.471 (0.001) | ↓ | 0.471 (0.001) | 0.001 | 0.21 – 0.67 |
| Lipid indices | ||||||
| TC | 0.510 (<0.001) | 0.510 (<0.001) | ↓ | 0.510 (<0.001) | 0.001 | 0.26 – 0.70 |
| TG | 0.510 (<0.001) | 0.511 (<0.001) | ↑ | 0.511 (<0.001) | 0.001 | 0.26 – 0.70 |
| LDL-c | 0.510 (<0.001) | 0.496 (<0.001) | ↓ | 0.496 (<0.001) | 0.001 | 0.24 – 0.69 |
| HDL-c | 0.510 (<0.001) | 0.522 (<0.001) | ↑ | 0.522 (<0.001) | 0.001 | 0.28 – 0.71 |
| TYGI | 0.510 (<0.001) | 0.497 (<0.001) | ↓ | 0.497 (<0.001) | 0.001 | 0.24 – 0.69 |
Impact of lipid and glycemic indices on the relationship between thyroid peroxidase antibodies with free triiodothyronine hormone by using zero-order (or none) and partial correlations.
| Controllers | Zero order correlation coefficient | Partial correlation coefficient | Changes in correlation coefficient | r ( | Adjusted | 95 % CI for r |
|---|---|---|---|---|---|---|
| Age | -0.553 (<0.001) | -0.545 (<0.001) | ↓ | -0.545 (<0.001) | 0.001 | -0.73 – -0.32 |
| BMI | -0.553 (<0.001) | -0.511 (<0.001) | ↓ | -0.511 (<0.001) | 0.001 | -0.72 – -0.31 |
| Glycemic indices | ||||||
| FSG | -0.553 (<0.001) | -0.560 (<0.001) | ↓ | -0.553 (<0.001) | 0.001 | -0.73 – -0.32 |
| HbA1c (%) | -0.553 (<0.001) | -0.518 (<0.001) | ↑ | -0.518 (<0.001) | 0.001 | -0.70 – -0.28 |
| Lipid indices | ||||||
| TC | -0.553 (<0.001) | -0.560 (<0.001) | ↑ | -0.560 (<0.001) | 0.001 | -0.73 – -0.33 |
| TG | -0.553 (<0.001) | -0.597 (<0.001) | ↑ | -0.597 (<0.001) | 0.001 | -0.76 – -0.37 |
| LDL-c | -0.553 (<0.001) | -0.519 (<0.001) | ↓ | -0.519 (<0.001) | 0.001 | -0.70 – -0.28 |
| HDL-c | -0.553 (<0.001) | -0.557 (<0.001) | ↑ | -0.577 (<0.001) | 0.001 | -0.73 – -0.33 |
| TYGI | -0.553 (<0.001) | -0583 (<0.001) | ↑ | -0.583 (<0.001) | 0.001 | -0.75 – -0.36 |
Impact of lipid and glycemic indices on the relationship between thyroid peroxidase antibodies with free thyroxine hormone by using zero-order (or none) and partial correlations.
| Controllers | Zero order correlation coefficient | Partial correlation coefficient | Changes in correlation coefficient | r ( | Adjusted | 95 % CI for r |
|---|---|---|---|---|---|---|
| Age | -0.406 (0.005) | -0.386 (0.008) | ↓ | -0.397 (0.006) | 0.0077 | -0.61 – -0.31 |
| BMI | -0.406 (0.005) | -0.363 (0.013) | ↓ | -0.397 (0.006) | 0.0077 | -0.59 – -0.11 |
| Glycemic indices | ||||||
| FSG | -0.406 (0.005) | -0.395 (0.007) | ↓ | -0.401 (0.007) | 0.0079 | -0.61 – -0.13 |
| HbA1c (%) | -0.406 (0.005) | -0.339 (0.021) | ↓ | -0.392 (0.008) | 0.008 | -0.61 – -0.12 |
| Lipid indices | ||||||
| TC | -0.406 (0.005) | -0.406 (0.005) | ⭤ | -0.411 (0.005) | 0.0077 | -0.62 – -0.14 |
| TG | -0.406 (0.005) | -0.418 (0.004) | ↑ | -0.425 (0.004) | 0.0077 | -0.63 – -0.16 |
| LDL-c | -0.406 (0.005) | -0.396 (0.006) | ↓ | -0.398 (0.006) | 0.0077 | -0.61 – -0.13 |
| HDL-c | -0.406 (0.005) | -0.421 (0.004) | ↑ | -0.417 (0.005) | 0.0077 | -0.62 – -0.15 |
| TYGI | -0.406 (0.005) | -0.420 (0.004) | ↑ | -0.406 (0.005) | 0.0077 | -0.62 – -0.14 |
Additionally,
Multiple linear regression model predicting serum TPO-Ab levels with 95 % confidence intervals for β-coefficients.
| Predictor | β-coefficients | Approx. SE | 95 % CI | Interpretation |
|---|---|---|---|---|
| HbA1c (%) | 3.259 | 1.63 | 0.06 – 6.46 | Strongest positive trend |
| BMI (kg/m²) | 1.206 | 0.82 | –0.40 – 2.81 | Mild positive effect |
| TyG index | 0.733 | 0.55 | –0.34 – 1.81 | Positive but broad CI |
| Age (years) | –0.266 | 0.18 | –0.62 – 0.09 | Weak negative |
| FSG (mg/dL) | –0.012 | 0.009 | –0.03 – 0.01 | Negligible |
| TG (mg/dL) | –0.055 | 0.03 | –0.11 – 0.00 | Borderline negative |
| LDL-c (mg/dL) | –0.087 | 0.06 | –0.21 – 0.04 | Non-significant negative |
| HDL-c (mg/dL) | 0.232 | 0.15 | –0.06 – 0.52 | Weak positive |
Change in correlation coefficients (Δr) between TPO-Ab and thyroid hormones after simultaneous adjustment for all covariates.
| Hormone | Zero-order r | Partial r (all covariates) | Δr | 95 % CI for Δr |
|---|---|---|---|---|
| TSH | 0.510 | 0.462 | –0.05 | –0.34 – 0.45 |
| FT3 | –0.553 | –0.501 | 0.05 | –0.44 – 0.38 |
| FT4 | –0.406 | –0.358 | 0.05 | –0.42 – 0.39 |
Multivariate Regression Analysis
A multivariate linear regression model was developed to identify independent predictors of serum TPO-Ab levels, with age, BMI, glycemic indices, and lipid parameters included as covariates. The overall model accounted for 29.1 % of the variance in TPO-Ab concentrations (R² = 0.291); however, it did not reach statistical significance (F = 1.685, p = 0.128).
Among the predictors, HbA1c (%) exerted the strongest positive association (β = 3.259), followed by BMI (β = 1.206) and the TyG index (β = 0.733). Conversely, age (β = –0.266) and fasting serum glucose (β = –0.012) were inversely associated with TPO-Ab levels. Regarding lipid indices, HDL-c demonstrated a modest positive association (β = 0.232), whereas TG and LDL-c showed inverse relationships (β = –0.055 and β = –0.087, respectively). Figure 1 presents the multivariate regression coefficients with 95 % confidence intervals for all predictors of TPO-Ab levels.

Multivariate regression coefficients with 95% confidence intervals for predictors of TPO-Ab levels. (r=0.539, R2=29.1, F=1.685, p=0.128).
DISCUSSION
This study investigated preliminary associations between thyroid peroxidase antibodies (TPO-Ab) and thyroid hormones in patients with autoimmune thyroid disease (AITD) receiving levothyroxine (LT4). We evaluated the potential modifying effects of demographic, anthropometric, glycemic, and lipid variables on these associations. Our data indicate that TPO-Ab concentrations are positively correlated with serum thyroid-stimulating hormone (TSH), whereas negative associations were observed with free triiodothyronine (FT3) and free thyroxine (FT4). These correlations weakened after adjustment for age, body mass index (BMI), glycemic markers, and low-density lipoprotein cholesterol (LDL-c), but unexpectedly strengthened after adjustment for triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), and the triglyceride–glucose (TyG) index. Although the overall regression model did not reach statistical significance (F = 1.685, p = 0.128), the direction and relative magnitude of the β-coefficients support possible relationships involving metabolic factors—particularly glycated hemoglobin (HbA1c), BMI, and TPO-Ab.
The positive association between TPO-Ab titers and serum TSH detected in this study corroborates earlier findings linking thyroid autoimmunity with diminished thyroid function. Shimizu et al. demonstrated that individuals with higher TPO-Ab concentrations exhibited increased TSH, particularly among women, thereby underscoring the role of autoantibodies in thyroid dysfunction 12. Likewise, Al-Rabia 13 reported significant correlations between TPO-Ab and TSH, FT3, as well as FT4 in patients with autoimmune thyroid disease. Notably, the magnitude of the association in our cohort exceeded that observed in some previous studies, a discrepancy that may reflect differences in patient selection, disease stage, or the confounding influence of LT4 therapy.
Our results suggest that adjustment for BMI and glycaemic indices attenuates the observed association between TPO-Ab and thyroid hormones. This observation aligns with the findings of Ostrowska et al., who reported that weight reduction in patients with Hashimoto’s thyroiditis decreased TPO-Ab concentrations and improved thyroid hormone homeostasis 4. Likewise, Mazaheri et al. showed that insulin resistance and disturbances in glycaemic control were associated with altered TPO-Ab levels in hypothyroid patients receiving thyroxine replacement therapy 14. Collectively, these data indicate that obesity and glycaemic dysregulation may modulate antibody–hormone interactions. Nevertheless, the relatively small changes in correlation coefficients (Δr < 0.05) suggest that the biological significance of this attenuation is limited.
Interestingly, lipid parameters, particularly triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C), modestly magnified the observed trends. Although this pattern suggests a potential interaction between lipid metabolism and thyroid autoimmunity, the corresponding changes in correlation coefficients (r) were small (generally < 0.02), indicating that the direction of association is theoretically intriguing yet of uncertain biological relevance. One possible explanation is that levothyroxine (LT4) therapy, which is known to influence lipid turnover, may have indirectly contributed to these associations. Wu et al. reported a positive relationship between thyroid peroxidase antibody (TPO-Ab) titers and metabolic disorders, including dyslipidemia, in a large euthyroid population, with stronger associations in women than in men 15. Likewise, Kumar et al. observed that subclinical hypothyroidism with positive TPO-Ab was frequently associated with elevated cholesterol, TG, and low-density lipoprotein cholesterol (LDL-C) levels 16. The paradoxical amplification noted in our study might therefore reflect underlying metabolic compensation mechanisms rather than a direct causal link.
To assess how simultaneous covariate adjustment affected the observed associations, we calculated the change in correlation magnitude (Δr) between zero-order and fully adjusted partial correlations (
The regression model in this study suggested a potential contribution of HbA1c and BMI to TPO-Ab variability, consistent with previous evidence that links metabolic status to thyroid autoimmunity. However, the model's relatively low explanatory power (29.1 %) indicates that additional genetic, environmental, and immunological determinants probably influence TPO-Ab levels. Previous studies have reported that age and smoking also affect the antibody–hormone relationship 17,18, supporting our observation that adjustment for age attenuated the association between TPO-Ab and thyroid function tests. Moreover, the retention of statistical significance after false-discovery-rate adjustment implies that the observed relationships between TPO-Ab and thyroid hormones are unlikely to be false positives but rather represent authentic biological associations.
These findings provide preliminary yet clinically meaningful insights. They emphasize the necessity of adjusting for metabolic and demographic covariates when examining the association between thyroid autoantibodies and hormone concentrations; failure to do so can lead to systematic bias and misinterpretation. Notably, the counter-intuitive behaviour of lipid parameters indicates that lipid metabolism may play a direct pathophysiological role in thyroid autoimmunity, and that levothyroxine (LT4) therapy could further modulate this interplay.
This study has several limitations. First, we did not perform a quantitative assessment of iodine status; however, national surveillance data suggest adequate dietary iodine intake in the study area. Second, the relatively small sample size and single-center design may restrict the external validity of our findings. Third, quantitative information on LT4 dose (μg·kg⁻¹·day⁻¹) was unavailable, and inter-individual dose variation was therefore not considered in the statistical models. Because higher LT4 doses can modulate lipid metabolism by up-regulating hepatic LDL-receptor expression and lowering circulating triglyceride concentrations, LT4 dose may represent an unmeasured confounder in the observed lipid associations, as previously reported by Unnikrishnan et al. 19. Moreover, we did not collect information on dietary habits or physical activity, and the cross-sectional design precludes causal inferences between metabolic parameters and thyroid autoimmunity. Finally, the subject-to-predictor ratio was relatively low (5 : 1), potentially diminishing the statistical power of the multivariable analyses. Nevertheless, acceptable multicollinearity diagnostics (VIF < 2) and uniform coefficient directions support the robustness of the presented models. Consequently, the current results should be considered hypothesis-generating and require confirmation in larger, multicenter, longitudinal studies.
CONCLUSION
This investigation demonstrates that the association between thyroid-peroxidase antibodies (TPO-Ab) and thyroid hormones in autoimmune thyroid disease is substantially influenced by demographic, anthropometric, glycaemic, and lipid variables. Whereas age, an elevated body-mass index (BMI), and adverse glycaemic indices attenuate antibody–hormone correlations, lipid parameters—particularly triglycerides and high-density lipoprotein cholesterol (HDL-c)—paradoxically strengthen these interactions. Multivariate regression further identified glycated haemoglobin (HbA1c) and BMI as the principal predictors of TPO-Ab variability, although the overall explanatory capacity remained limited.
These preliminary data highlight the importance of considering metabolic and demographic covariates when interpreting thyroid antibody concentrations in both clinical practice and research. Employing partial, rather than simple, correlation analyses provides a more rigorous framework for understanding antibody–hormone interplay and reduces the risk of bias from confounding factors. Clinically, this approach may refine the assessment and management of autoimmune thyroid disease by integrating metabolic risk factors into patient-specific treatment strategies.
Abbreviations
AITD: Autoimmune Thyroid Disease; BMI: Body Mass Index; CI: Confidence Interval; CLIA: Chemiluminescent Immunoassay; CV: Coefficient of Variation; FDR: False Discovery Rate; FSG: Fasting Serum Glucose; FT3: Free Triiodothyronine; FT4: Free Thyroxine; HbA1c: Glycated Hemoglobin; HDL-c: High-Density Lipoprotein Cholesterol; HPLC: High-Performance Liquid Chromatography; LDL-c: Low-Density Lipoprotein Cholesterol; LT4: Levothyroxine; SD: Standard Deviation; TC: Total Cholesterol; TG: Triglycerides; TG-Ab: Thyroglobulin Antibodies; TPO-Ab: Thyroid Peroxidase Antibodies; TSH: Thyroid-Stimulating Hormone; TyG Index: Triglyceride-Glucose Index; VIF: Variance Inflation Factor
Acknowledgments
The authors would like to express their sincere gratitude to the administration of Anwar Sheikha Medical City for facilitating the conduct of this study and providing access to clinical facilities. Special thanks are extended to the staff of the AMC laboratory for their invaluable assistance in sample collection and biochemical analyses. The authors also acknowledge the patients who generously agreed to participate in this research.
Author’s contributions
Rafl M. Kamil conceptualized and designed the study, supervised the research, conducted the data analysis and interpretation, and drafted and critically revised the manuscript. Najmaddin S. H. Khoshnaw contributed to patient recruitment, clinical data acquisition, and ethical coordination. Raneen Subhi Atiyah assisted with data collection, laboratory coordination, and preliminary statistical analysis. Mirwan Malek Kamil and Muhammad Malik Kamil contributed to data management and data collection. Hassan Haider Al Salamy contributed to laboratory analyses and methodological support. All authors reviewed, edited, and approved the final manuscript.
Funding
None.
Availability of data and materials
An anonymized dataset can be made available from the corresponding author on reasonable request for academic, non-commercial research purposes, subject to institutional and ethical approval.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

