Romiplostim: Successful treatment of a pregnant woman with refractory immune thrombocytopenia: A case report and literature review
- Department of Hematology and Medical Oncology, Kermanshah University of Medical Sciences, Kermanshah, Iran
- Department of Hematology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- Department of Genetics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
- Department of Biology, School of Sciences, Razi University, Kermanshah, Iran
Abstract
Immune thrombocytopenia is characterized by reduced platelet count. This condition occurs in both adults and children. The most common form of thrombocytopenia is primary ITP and autoantibodies are involved in its development. In this study, our patient was a pregnant woman with ITP who showed refractory to prednisolone and splenectomy as first and second line treatment, respectively, but the response to treatment with Romiplostim and platelet count was favorable, and delivery was reported without fetal complications.
Background
Immune thrombocytopenia (ITP), formerly known as idiopathic thrombocytopenic purpura, is an autoimmune disease described by a severe reduction in peripheral blood platelets 1. In thrombocytopenia, the platelet count reaches less than 100 x 10/L 2. Adult ITP has an incidence about 2.0 to 4.0 per 100,000, while in childhood is 1.9 to 6.4 per 100,000 34. The major complications for patients with ITP are bleeding and intracranial hemorrhage 5. Types of thrombocytopenia include primary and secondary ITP. Primary ITP is due to the autoimmune destruction of platelets, which include 80% of the patients. Secondary ITP is related to other conditions such as lupus erythematosus, infections such as HCV and HIV, and lymphoma 6. Also, the acute form is a temporary condition that lasts up to 3 months and mainly affects children, while the chronic form may persist for more than 12 months and affect adults 7. For patients with chronic ITP, there are several treatments that includes corticosteroids, immunoglobulin, splenectomy, and thrombopoietin receptor agonists (TPO-RAs). TPO-RA is commonly used in patients who do not respond adequately to corticosteroids and splenectomy 5. The rationale for selecting TPO-RAs in the treatment of refractory ITP is that the level of thrombopoietin (TPO) is abnormal or low in the patients. Also, the response rates of ITP patients to IVIG and corticosteroids have been reported to be less than 40% 8, while for TPO-RAs, the response rate is reported 59-88% 9. In this study, we have reported a pregnant woman with ITP who was refractory to prednisolone and splenectomy but responded positively to the treatment with Romiplostim.
Case History
A 22-year-old woman with symptoms such as weight loss, petechial rashes on parts of the body and bleeding from the gums and nose, referred to the Kermanshah Hematology and Oncology Clinic in May 2015. There was no suspicious case in the patient's clinical history. During her examinations, multiple petechial rashes were observed on the organs of the body and spleen was not palpable. Also, laboratory studies showed decreased platelet count and positive anti-dsDNA. The results of complete blood count are as follows: platelet count: 19 x10/L (normal range: 120–450), hemoglobin (Hb): 9 g/dL (normal range: 11.5–18.5), white blood cell (WBC): 10 x 10/ L (normal range: 3.5–11). Therefore, initial treatment with Prednisolone (1 mg/kg/day) and Hydroxychloroquine (200 mg/day), was performed for several months, and the results of complete blood count (CBC) were as follows:
Platelet count: 45 x 10/ L, Hb: 11.6 g/dl, WBC: 12.3 x 10/ L, anti-dsDNA: positive. After several cycles of treatment, CBC results showed refractory to treatment in the patient, and the physician asked for bone marrow aspiration (BMA). The BMA results showed the normal number of megakaryocytes with less platelet attachment. Therefore, the final diagnosis was ITP. Accordingly, the patient's treatment continued with IVIG (intravenous immunoglobulins) (15 g/day), and also due to the presence of lupus, Hydroxychloroquine and Azathioprine (150 mg/day) were used. After 4 months, due to the results of relatively low platelet count and splenomegaly in the patient, the first line treatment failed, and in the next action, she was subjected to splenectomy.
Shortly after splenectomy, the patient suddenly decided to be pregnancy. Therefore, according to the patient's condition, treatment with steroid regimens was performed in the first trimester of pregnancy. Also, abdominal ultrasound indicated the progression of normal pregnancy; however, the platelet count did not increase significantly. Since the patient was passing pregnancy and approaching delivery time, the decision was taken to use Romiplostim for her. After three cycles of treatment, the response to treatment was good, and her platelet count was about 164 x 10/L. In the parturition, the platelet count was 75 x 10/ L, and she gave birth to a healthy baby. After delivery, the patient's platelet count was still good, and now the treatment with romiplostim continues for her and the patient is in favorable condition.
Discussion
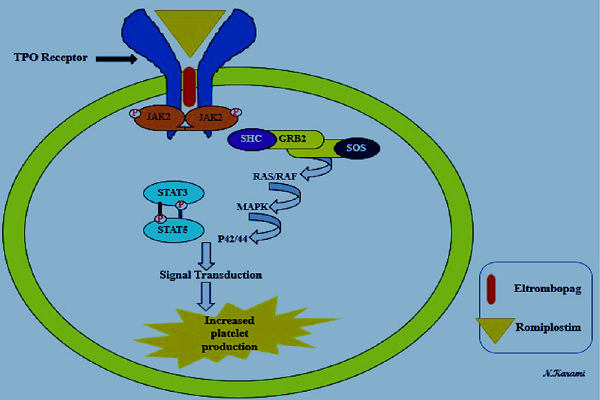
Patients with ITP have specific characteristics, including platelet count <100 × 10 / L and bleeding. Increased platelet destruction and reduced platelet production are two main mechanisms of ITP pathogenesis 10. In most patients, it seems that platelet destruction is due to the production of autoantibodies and is considered as the most common cause of ITP development 11. The glycoprotein IIa / IIIa on platelets is a target for autoantibodies 4. This mechanism is associated with platelet phagocytosis, and splenic macrophages and neutrophils are involved. Also, there are two factors involved in reducing platelet production: dysfunction of megakaryocytes and the inadequate level of TPO 5. In pregnant women, the incidence of ITP is 7% and the probability of occurrence of pregnancy-related disorders, such as stillbirth, pregnancy loss, premature delivery, and congenital anomalies, is relatively high 12. So, treatment options for these patients are very important. There are three lines of therapies for these patients: In the first line treatment, corticosteroids, IVIG, and anti-D immunoglobulins are approved 13. These factors are used, but due to the limited time of response, they are not considered for long periods. Corticosteroids (such as prednisolone) also decrease the autoantibodies production and inhibit the platelet destruction caused by macrophages 14, while IVIG reduced platelet destruction through binding to Fc receptors in the reticuloendothelial system 15. Splenectomy and Rituximab are considered as the main options in the second line treatment. For patients who have failed treatment with corticosteroids, splenectomy is usually recommended 16. Also, Rituximab, as an antibody, results in the inhibition of B cells by binding to CD20 17. In patients with chronic ITP who have been refractory to chemotherapy regimens such as corticosteroids, immunoglobulin or splenectomy, the FDA has been approved using TPO-RAs, such as Eltrombopag and Romiplostim (Nplate) 18. These are considered the third line treatment. Typically, megakaryocytes are as the source of platelets, and a chief regulator for the production of new platelets from megakaryocytes is TPO 19. The treatment of ITP with TPO-RAs is performed by activating TPO receptors of megakaryocytes in bone marrow. The JAK2/STAT5 and MAPK are the main pathways for the performance of TPO-RAs that enhance platelet count by inducing transcription of the genes involved in platelet proliferation (Figure 1) 20.

Cellular mechanism of Thrombopoietin Receptor Agonists (TPO-RAs). Eltrombopag and Romiplostim, as representatives of TPO-RAs, link to Thrombopoietin receptors and activate JAK2/STAT5 and MAPK pathways. These pathways induce transcription of the genes involved in platelet production, which leads to an increase in platelet count.
Nplate is the commercial form of romiplostim and showed an effective response in both splenectomized and nonsplenectomized patients 21. The safety and effectiveness in increasing and maintaining the platelet count and limited side effects are the benefits of romiplostim 4. The advantages and adverse effects of using romiplostim are presented in
Advantages and adverse effects of Romiplostim(
| Romiplostim | |
| Advantages | Adverse effects |
| Response rates ≈ 95% | Headache |
| Approved by the FDA for patients with chronic ITP | Fatigue |
| Increases quality of life | Diarrhea |
| Decreases the need for rescue treatments | Nasopharyngitis |
| Decreases bleeding complications | Hypoglycemia (in isolated cases) |
| Discontinue treatment and maintain the platelet counts (in isolated cases) | Bone-marrow fibrosis (in isolated cases) |
In the past years, due to a lack of human studies and adverse effects in animal studies, it was recommended that romiplostim should not be used routinely during pregnancy. However, in a few studies, the treatment of ITP patients with romiplostim has been reported during pregnancy without fetal complications 2526. In a case study, ITP was successfully treated in pregnant woman with systemic lupus erythematosus using romiplostim. After delivery, results of CBC showed a high platelet count (202 × 10/L) 26. In the similar study, the patient who has failed in first-line treatment showed an effective response to the drug 25. Similarly, Samuelson and colleagues reported two ITP patients who received romiplostim during pregnancy without severe complications to the mother or the fetus. In the first patient, the use of romiplostim increased the platelet count to 200 × 10/L, and in the second patient, the platelet count reached 184 × 10/L 23.
Also, recombinant human thrombopoietin (rhTPO) is a glycosylated form of TPO that expressed in Chinese hamster ovary cells. The rhTPO, as first-line therapy for refractory ITP, was approved by the China State Food and Drug Administration 27. In the latest study, rhTPO was used for ITP during pregnancy. This study was conducted in the Chinese population, and 74.2% of the patients responded to treatment with rhTPO 28. Finally, it's important to note that if TPO-based treatments are fully confirmed in terms of safety and efficacy, management of ITP will be facilitated in pregnant women. Our patient has the unique characteristics listed below:
-Pregnancy during treatment
-Presence of two types of main and accessory spleen
-Refractory to first and second line treatment
-A positive response to treatment using romiplostim without fetal complications
-Successful delivery
Conclusion
Regarding the risks of ITP for pregnancy, especially hemorrhage, the type of treatment is very imperative in these patients. Because romiplostim directly affects megakaryocytes in bone marrow, it seems to be a good option for the patients who were refractory to the first and second line treatment.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CCBY4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
ITP: Immune thrombocytopenia
CBC: complete blood count
BMA: bone marrow aspiration
TPO: thrombopoietin
IVIGs: intravenous immunoglobulins
TPO-RAs: thrombopoietin receptor agonists
rhTPO: recombinant human thrombopoietin
Ethics approval and consent to participate
Not to be applied.
Competing interests
The authors declare that they have no financial or other conflicts of interest.
Funding
None.
Authors’ contributions
Mehrdad Payandeh & Noorodin Karami: Literature search, Clinical studies, Data acquisition, Data analysis;
Noorodin Karami: Manuscript preparation, Manuscript review,
Guarantor; Afshin Karami: Concepts, Design, Definition of intellectual content, Literature search, Manuscript editing.
Jafar Barati Masgareh: Manuscript editing.

