
Hepatoprotective and nephroprotective effects of Trigonella foenum-graecum L. (Fenugreek) seed extract against sodium nitrite toxicity in rats
- Department of Physiology, Faculty of Veterinary Medicine, University of Kafkas, Kars – Turkey
- Department of Health Care Services, Atatürk Vocational School of Health Services, University of Kafkas, Kars – Turkey
- Department of Pathology, Faculty of Medicine, University of Çanakkale Onsekiz Mart, Çanakkale – Turkey
Abstract
Introduction: Feeding habits and environmental factors may rival genetic susceptibility as etiological factors related to various cancers. Humans are continuously exposed to many synthetic food additives, one of which is sodium nitrite (NaNO2). There is a direct correlation between increases in consumption of nitrite-treated products and incidence of tissue damage, hepatotoxicity, nephrotoxicity and some types of cancer. The objective of this study was to investigate the protective effects of Trigonella foenum-graecum (TFG) on NaNO2-induced hepatotoxicity and nephrotoxicity.
Methods: Forty rats were randomly assigned (10 per group) to control (physiological saline solution), TFG (150 mg/kg/day), NaNO2 (80 mg/kg/day), and NaNO2+TFG (80 mg/kg/day + 150 mg/kg/day) groups. This group was offered TFG seed extract two hours before NaNO2. At the end of three months, the rats were decapitated, and blood, kidney and liver tissues were removed.
Results: Three months of oral administration of NaNO2 increased serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea, creatinine, and pro-inflammatory cytokine levels in the liver and kidney tissues [except for liver Interleukin 1 alpha (IL-1a)] of rats. Serum AST, ALT, urea, creatinine, liver IL-6, and kidney tumor necrosis factor-a (TNF-a), IL-6, IL-1a levels significantly decreased in the NaNO2+TFG group compared to the NaNO2 group. Pathological examinations, it was determined show that exogenously administered TFG could alleviate the effects of NaNO2 hepatotoxicity and nephrotoxicity.
Conclusions: Our results suggest that exogenous TFG mitigates NaNO2-administration induced hepatotoxicity and nephrotoxicity. TFG extract exerted antioxidative and anti-inflammatory effects, and played a significant role in preventing hepatic and renal damage induced by chronic NaNO2 administration.
Introduction
Much research has been devoted to determining the relationship between diet and chronic diseases like diabetes, cancer, coronary heart disease, metabolic syndrome and osteoporosis. Humans are continuously exposed to many synthetic food additives, one of which is sodium nitrite (NaNO). NaNO inhibits bacterial growth secondary to colour preservatives, improves flavour, and extends the shelf-life of meat and fish products1,2. Unfortunately, high levels of NaNO intake may increase the risks of cancer, hepatotoxicity, nephrotoxicity, inflammation, oxidative stress, and tissue injury3,4. The toxic effects of NaNO are due to the production of nitrosamines and nitroamides when nitrites react with amines and amides in food5,6,7. The resulting nitrosamines and nitroamides also increase the production of free radicals8. Intestinal bacteria with nitrite reductase activity may increase the production of nitric oxide (NO) from nitrites9,10. Akhzari . (2019) reported that alanine aminotransferase (ALT), alkaline phosphatase (ALP), caspase-3 activities, malondialdehyde (MDA), TNF-α and transforming growth factor-β1 levels increased and reduced glutathione (GSH), glutathione reductase (GR) and glutathione peroxidase (GPx) levels decreased in rats treated with sodium nitrite11. Another study determined that NaNO increased lipid peroxidation, MDA, NO levels, arginase activity but decreased activity of GSH and catalase (CAT) toxicity induced by sodium nitrite in rats6. Also, Ansari (2019) indicated that NaNO-induced oxidative stress causes a decrease in the activity of antioxidant defence system, brush border membrane and metabolic enzymes in kidneys12.
There is a growing interest in the use of plant-derived agents for treating diseases and/or preventing chronic health conditions. L. (fenugreek or TFG) is an annual plant that belongs to the Leguminosae family13. Its seeds and leaves are commonly used as a condiment and seasoning, and a wheat and maize flour supplement for bread-making. Additionally, TFG is a staple food in Asian and North African regions14 and is also used in traditional medicine owing to its antidiabetic, hypoglycaemic, antioxidant, hypolipidemic and immunomodulatory effects15,16. The most important phytochemicals isolated from TFG are saponins, trigonelline alkaloids, trigocoumarin, phosphates, potassium, proteins (4-hydroxyisoleucine), choline, vitamin C, beta-carotene, nicotinic acid, and folic acid17,15. TFG may mitigate the adverse effects of NaNO administration. This study investigate the protective effects of TFG on NaNO-induced hepatotoxicity and nephrotoxicity were investigated.
Methods
Extraction of plant material
TFG seeds were purchased at a local herbal market. Mature fruits were collected between August and September and then sundried. After this process the seeds become yellow and light brown. Each seed is4-6 mm long and 2-3 mm width. They are slightly rough, squared or rectangular and unevenly shaped. We pulverated 1000 g of the seeds into a fine powder and dissolved this powder into a mixture of ethyl alcohol and water (2:8) at a ratio of 1:5 (seed weight: solvent volume)18. The mixture was kept at room temperature in a shaking water bath for 2 days. It was filtered through filter paper, and the excess solvents were evaporated under pressure at 50°C. We obtained 9 g of the extract from 250 ml of macerated seeds.
Experimental design
We used 40 female Wistar Albino rats, aged around 2 months. The rats were divided equally into four groups (10 rats per group) for the experiments. The animals were maintained under standard conditions [temperatures (23±2ºC), humidity (55±5%) and 12 hrs light: 12 hrs dark cycle] and were fed standard laboratory chow (Moisture: 12.8%, crude protein: 23.1%, crude cellulose: 5%, crude oil: 2.8%, crude ash: 7.1%, and sodium 0.60%). The rats were allowed tap water throughout the experiment. The animal protocol was accepted by the ethical committee for Animal Experiments at Kafkas University.
Control group (C): This group received oral physiological saline solution (300 µL/day).
group (TFG): This group received 150 mg/kg/day TFG seed extract dissolved in water.
Sodium nitrite group (NaNO): This group received 80 mg/kg/day NaNO dissolved in water.
Sodium nitrite + group (NaNO+TFG): This group received 150 mg/kg/day TFG seed extract 60 minutes prior to 80 mg/kg/day NaNO.
Rats were administered with NaNO, TFG extract and physiological saline solution by oral gavage over three months. The dose and duration of NaNO and TFG extract used in this study were in the range of those used in other studies, and of the same animal species19,20,21.
Biochemical analysis and Histopathological evaluations
At the end of the experiment, blood samples were collected under 0.4 ml/kg pentobarbital sodium anaesthesia via the intracardiac route. Blood samples were taken with a non-anticoagulant tube and then centrifuged at 1000xg for 15 min at 4°C to produce serum. The serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) (Mindray BS 120), urea and creatinine levels (Architect c16000 Abbott Diagnostic–USA model) were measured using an autoanalyser. The rat livers and kidneys were removed quickly and cleaned with cold physiological saline solution. Pieces of the livers and kidneys were homogenised in a 10-fold volume of phosphate buffered saline. We used ELISA kits to determine pro-inflammatory cytokine (IL-6, IL-1α, TNF-α) levels in tissue homogenates (Elabscience WuHan-P.R.C). Moreover, some tissues were fixed in 10% formalin and processed according to standard procedures. We cut 4-μm-thick paraffin sections, stained them with haematoxylin-eosin and evaluated the results using a light microscope.
Statistical analysis
We used a one-way ANOVA to determine whether there were significant differences among the groups. The Tukey multiple range test was used to detect significant pairwise differences between the groups. A value of p<0.05 was considered significant. All statistical tests were performed using SPSS 18.
Results
We evaluated the hepatoprotective and nephroprotective effects of TFG seed extract by measuring the serum activities of ALT, AST, urea and creatinine. Rats administered NaNO showed significant increases in serum ALT compared to the C and TFG groups (p<0.05). We found a significant increase in the AST levels in the NaNO group relatively to the C and TFG groups (p<0.001). Moreover, there was a significant decrease in AST levels in the TFG group when compared to the C group (P<0.01). Administration of NaNO with TFG seed extract (NaNO2+TFG) significantly decreased serum AST and ALT levels compared to the NaNO group (p<0.001, p<0.05 respectively). A significant increase in urea levels were observed in the NaNO and NaNO+TFG groups compared to the C and TFG groups (p<0.001, p<0.01 respectively). Serum urea levels were also significantly lower in the NaNO+TFG group, compared to the NaNO group (p<0.05). Both the NaNO and NaNO+TFG groups exhibited significantly higher serum creatinine levels compared to the C and TFG groups (p<0.001, p<0.05 respectively). Moreover, the serum creatinine levels were significantly decreased in the NaNO+TFG group, as compared to the NaNO group (p<0.05) (Figure 1).
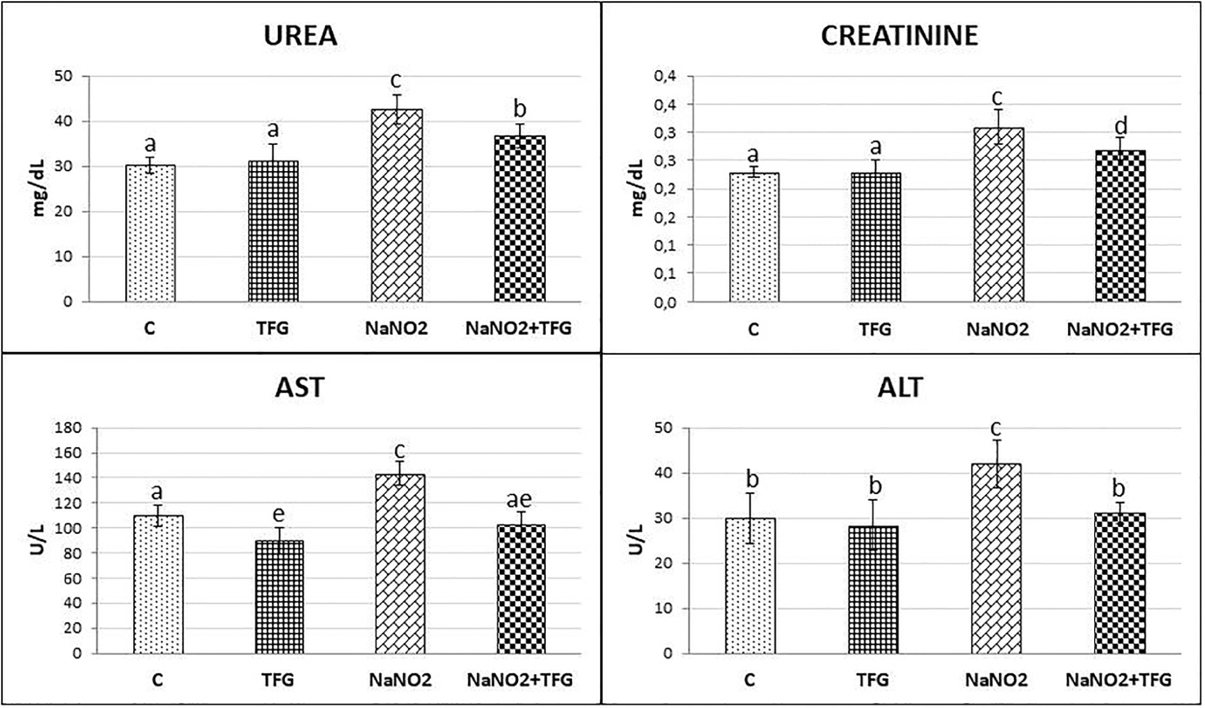
Effect of TFG extract on serum Urea (mg/dL) and Creatinine (mg/dL) levels, AST (U/L), ALT (U/L) mean±SD of animals in each group (n:10). a-b, a-e: p<0.01, a-c, c-e, c-ae: p<0.001, a-d, b-c, c-d: p<0.05.
NaNO significantly increased liver TNF-α and IL-6 levels compared to the C and TFG groups (p<0.05, p<0.001 respectively), yet, did not affect the IL-1α. Chronic treatment with TFG+NaNO significantly decreased IL-6 in liver tissues of rats, compared with the NaNO group (p<0.01). However, IL-6 increased in TFG+NaNO group compared to C group (p<0.05) (Figure 2). NaNO administration increased kidney TNF-α, IL-6 and IL-1α levels when compared to the control group (p<0.05, p<0.001 and p<0.01 respectively). Our results showed that the TFG+NaNO group exhibited significantly decreased kidney TNF-α and IL-6 levels comparing to with the NaNO group (p<0.05) (Figure 3).

Effect of TFG extract on liver proinflammatory cytokines (IL-6, IL-1α, TNF-α) levels (pg/mL), mean±SD of animals in each group. a-b:p<0.05, b-c: p<0.01, a-c, ab-c: p<0.001

Effect of TFG extract on kidney proinflammatory cytokines (IL-6, IL-1α, TNF-α) levels (pg/mL), mean±SD of animals in each group. a-b,b-e: p<0.05, a-d, ab-e: p<0.01, a-e: p<0.001.
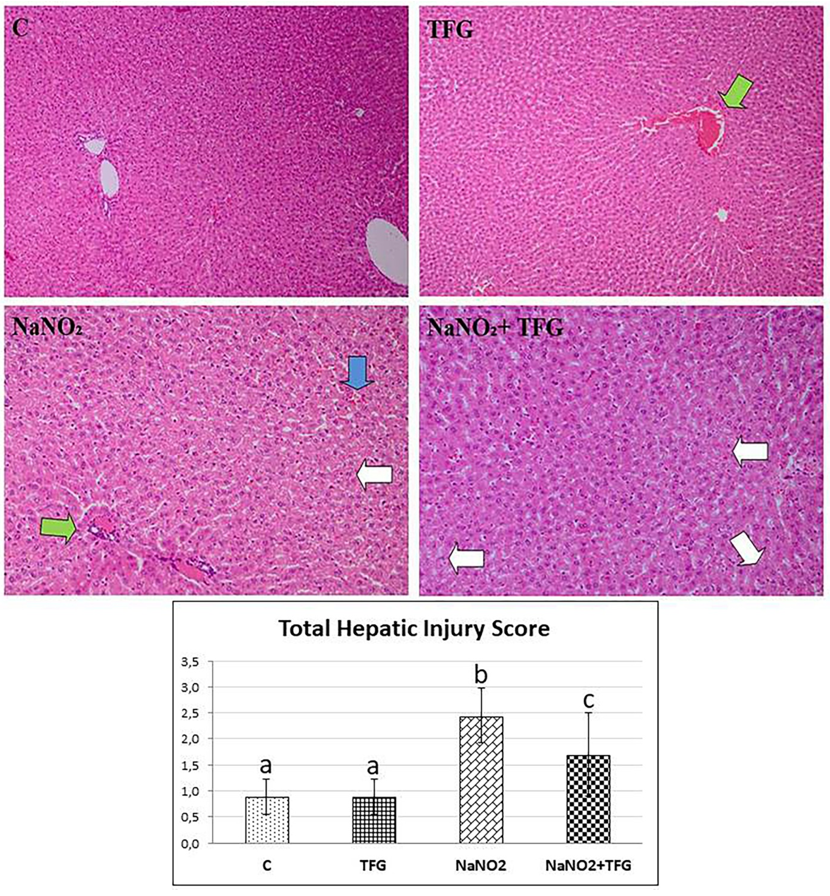
Focal moderate hepatocyte damage (white arrow), interhepatocytic hemorrhage (blue arrow) and vascular congestion (green arrow), H & E-200x. a-b:p<0.001, a-c, b-c: p<0.05
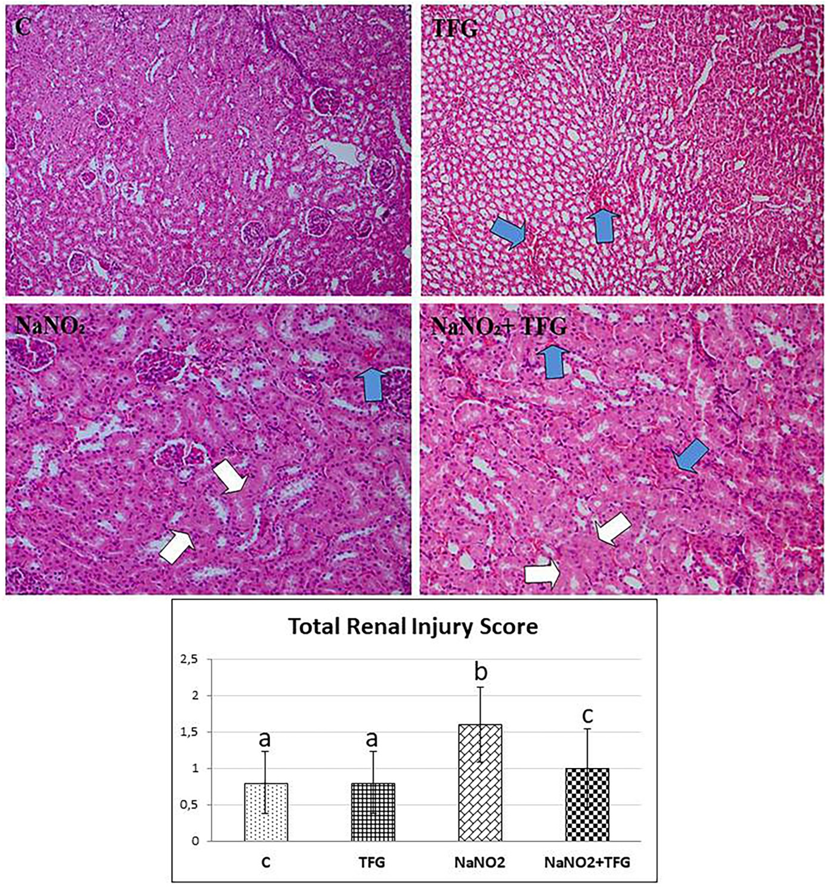
Tubular epithelial cell damage (white arrow) andinter tubular bleeding areas (blue arrow), H & E 200x. a-b: p<0.01, b-c: p<0.05, a-c: p>0.05
Histopathological examination revealed hepatocyte damage of variable prevalence and intensities in all NaNO group rats. Also, in the TFG+NaNO group, half of the rats had hepatocyte damage (Figure 4). In the C group, we observed mild congestion in only one kidney tissue sample. In the NaNO group, most of the cases had focal tubular epithelial damage, mild vascular congestion in one of these damaged cases and the least severe inter and intratubular haemorrhage in the damaged cases. There was one case of mild violent intratubular haemorrhage in the TFG group. In the TFG+NaNO group, mild severe vascular congestion and mild severe intermittent haemorrhage was detected in one of the cases with mild severe focal tubular epithelial damage in most of the cases (Figure 5). We therefore conclude that exogenously-administered TFG may alleviate the effects of NaNO hepatotoxicity and nephrotoxicity.
Discussion
NaNO is an important food additive with antibacterial properties for pathogens such as , O157:H7 and . However, because high doses and chronic NaNO consumption increase the levels of carcinogenic N-nitrosamines, restricted dietary intake should be considered25,26. Prolonged oral administration of NaNO has been shown to result in widespread and moderate inflammation and damage in rats. Previous studies support our findings2,4,27. However, oral administration of TFG+NaNO significantly reduced hepatic and renal damage and inflammation compared to the NaNO group. TFG mitigates various toxicities, but there is no published literature on the effects of TFG extract on NaNO toxicity. This study presented an important step in this regard. TFG extract exerted positive effects on alcohol28, acrylamide29, and cyclophosphamide + buthionine-SR-sulfoximine30 toxicity in previous studies. The seeds also have hypoglycaemic31, anti-inflammatory32, antiulcerogenic33, and antinociceptive34 effects. In another study, both hepatic and testicular dysfunction were found in male rats exposed to chronic cadmium, but the application of fenugreek seed powder was beneficial for these disorders35. Thirunavukkarasu (2003) found that aqueous extract of TFG may protect against damage to lipid peroxidation and liver and brain tissue damage36. Belaïd-Nouira (2013) found that treatment with fenugreek seed powder normalises plasma markers and alleviates histopathological changes in cases with aluminium-induced kidney damage37.
Serum enzymes including AST, ALT, urea and creatinine are used to evaluate patients with hepatic and renal disorders. This enzymes activity increases in cases of liver and kidney damage38. NaNO reacts with amines in the stomach, producing nitrosamines that cause serious damage to the liver and kidneys39. NaNO administration can alter liver and kidney function, as evidenced by significant increases in AST, ALT, urea and creatinine, compared to group C. TFG seed extract lowers levels of key enzymes that characterise liver and kidney damage.
NaNO initiates peroxidative damage in cells and increases the activation of pro-inflammatory cytokines40. Therefore, pro-inflammatory cytokines may play an important role in the formation of liver and kidney damage. Al-Gayyar (2014) found that NaNO increased levels of cardiac pro-inflammatory cytokines and decreased levels of anti-inflammatory cytokines1. Another study determined that NaNO caused renal toxicity and significantly increased oxidative stress, inflammation, and apoptosis markers41. In the present study, we determined that NaNO caused serious damage to liver and kidney tissues and led to significant increases in pro-inflammatory cytokine levels in these tissues. TFG seed extract reduced liver and kidney damage induced by NaNO, and reduced pro-inflammatory cytokine levels, especially in the kidneys. Previous studies showed that TFG seeds are a rich source of polyphenols. Polyphenols such as apigenin, kaempferol, quercetin, vitexin and tricine, were identified by HPLC42,43. Also, sapogenins, diosgenin, 4-hydroxy-isoleucine, trigonelline, alkaloids, phosphate, potassium, copper, protein, choline, vitamin C, beta carotene, nicotinic acid and folic acid are active chemical components of TFG44,45,46. Bin-Hafeez (2013) reported that the immunostimulatory activity of TFG is mediated by the saponins, fibres and flavonoids it contains15. The toxic effects of NaNO are caused by the production of nitrosamines and nitrosamides, which are known to cause tissue damage due to inflammation and oxidative stress. In this study, TFG extract was found to cause significant decreases in liver and kidney damage markers and pro-inflammatory cytokine levels in NaNO-induced hepatotoxicity and nephrotoxicity. It is proposed that TFG performs hepato and nephroprotective effects with immunostimulatory factors found in previous studies.
Conclusions
In conclusion, chronic NaNO consumption causes severe physiological and histopathological changes in liver and kidney tissues in rats. The TFG extract showed antioxidant and anti-inflammatory effects, and prevented hepatic and renal damage induced by chronic NaNO administration.
Abbreviations
ALP: Alkaline phosphatase
ALT : Alanine aminotransferase
AST : Aspartate aminotransferase
CAT: Catalase
GPx: Glutathione peroxidase
GR: Glutathione reductase
GSH: Reduced glutathione
IL-1α : Interleukin 1 alpha
IL-6 : Interleukin-6
MDA: Malondialdehyde
NO: Nitric oxide
TFG :
TNF-α : Tumor necrosis factor alpha
Competing Interests
The authors declare no conflict of interest.
Authors' Contributions
The design, experimental part and biochemical analysis of the study were carried out by Dr. Gözde ATİLA USLU and Dr. Hamit USLU. The histopathological evaluations were performed by Dr. Yasemen ADALI. All the authors took equal roles in writing the research.

