
The role of tumor-derived exosomes in tumor immune escape: A concise review
- Stem Cell Institute, University of Science Ho Chi Minh City, Viet Nam
- Laboratory of Stem Cell Research and Application, University of Science Ho Chi Minh City, Viet Nam
Abstract
Exosomes are small vesicles secreted by viable cells into the microenvironment. These vesicles bring various compositions, including lipids, RNAs and proteins, which carry information from producer cells to target cells. Cancer cells also produce exosomes, termed as tumor-derived exosomes (TDEs), which play important roles in immune modulation, angiogenesis and metastasis of tumors. This review summarizes the roles of TDEs in tumor immune escape mechanisms. TDEs affect all kinds of tumor-associated immune cells, including natural killer (NK) cells, dendritic cells (DCs), T and B lymphocytes, and myeloid-derived suppressor cells (MDSCs). Generally, TDEs suppress the immune system to promote tumor immune escape, thereby significantly contributing to tumorigenesis and metastasis.
Introduction
The environment surrounding cancer cells,. the local cellular environment in which tumors exist, is termed the tumor microenvironment (TME). The TME plays an important role in protecting tumors from the immune system1, as well as other pro-tumorigenic roles. The TME is surrounded by blood vessels, myofibroblasts, immune cells (such as monocytes, macrophages, T lymphocytes, B lymphocytes, myeloid cells, and natural killer (NK) cells), as well as non-cellular compositions (such as the extracellular matrix (ECM), signaling factors, and cytokines)1. Recently, in the TME, another non-cellular composition was discovered which has important roles in tumorigenesis and metastasis; this component was extracellular vesicles (EVs). The EVs in the TME is derived from several types of cells, including cancer cells and normal cells. There have been at least 3 types of EVs discovered in the TME: exosomes (derived from both cancerous and normal cells), microvesicles (derived from both cancerous and normal cells), and oncosomes (derived from cancerous cells) (Figure 1 ). To distinguish the exosomes from normal cells, exosomes secreted by the cells inside a tumor are termed tumor-derived exosomes (TDEs).
TDEs are involved in both development and metastasis of cancer via angiogenesis and immunosuppressive factors (which are carried within the vesicles)2. TDEs play a vital role of communication between cancer cells and the host environment in cancer metastasis1. Cancer cells also produce their own exosomes, term as cancer cell-derived exosomes (CDEs). In this concise review, we will just focus on the role of TDEs in tumor immune escape.
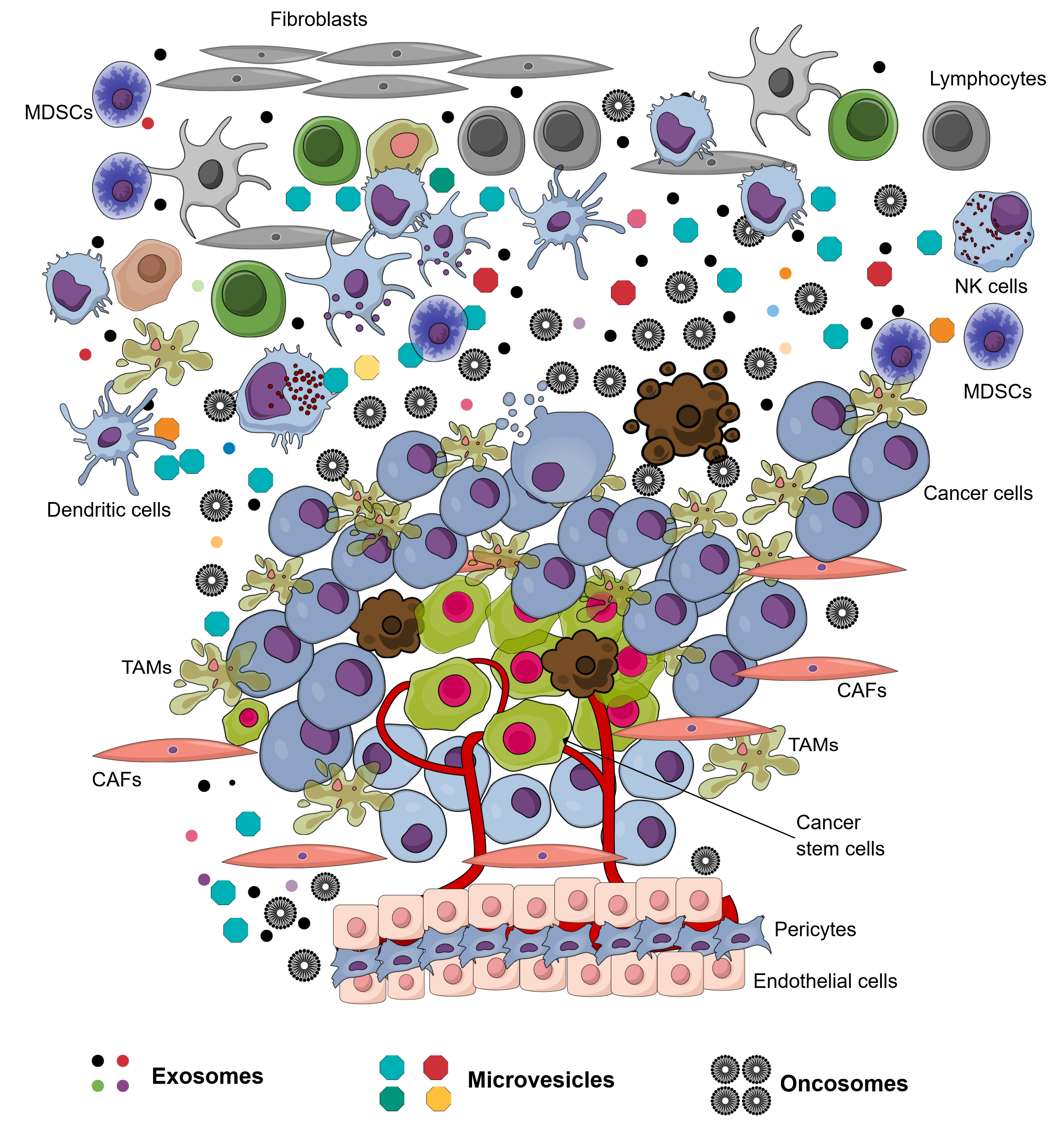
Tumor environment with various kinds of cells and their extracellular vesicles. Almost cells in this environment produce and secrete exosomes and microvesicles into the extracellular fluid. Some cancer cells produce oncosomes to contribute to this environment.
Tumor-derived exosomes (TDEs)
Exosomes are small membrane vesicles, ranging in size from 30-150 nm, and with a density of 1.10–1.14 g/ml. They are known to have a critical role in cell-cell communication. Due to the small dimensions, exosomes are visualized by electron microscopy3, 4, 5, 6, 2. In nature, exosomes were originally intraluminal vesicles released from late endosome multivesicular bodies (MVBs) after fusing with the plasma membrane4. Exosomes can be secreted by many cell types, including immune cells and cancer cells 2. The exosome structure consists of an outer lipid bilayer membrane, which encapsulates contents such as proteins, lipids, DNAs (. mtDNA, ssDNA, and dsDNA), RNAs ( lncRNAs, miRNAs, and mRNA), among other components. Exosomes exert their functions after delivery to or interaction with recipient cells3, 2.
The boundaries between markers and packaged components within an exosome are rather ambiguous. In essence, the TDEs may contain all the constituent components of the tumor, including those from the TME. Some typical proteins found on the surface of TDEs are tetraspanins, endosomal sorting complexes required for transport (ESCRT), membrane transport and fusion proteins, adhesive proteins, heat shock proteins, enzymes, receptor proteins, MHC I/II, integrins, PD-L1, EGFR, and TRAIL. On the contrary, in non-metastatic cells, the predominant proteins found are cell adhesion-related proteins2. Alix and TSG101 are molecular markers specific for exosomes that are related to multivesicular body (MVB) formation6, 2. Tetraspanins, flotillin-1, integrins, and MHC I/II represent several membrane proteins, while Hsp70, TSG101, and Alix are cytosolic proteins. Other proteins, as well as mRNA, miRNA, non-coding RNA, and DNAs, are found in exosomes, depending on the specific origin of those exosomes4.
The encapsulation of biological molecules into the exosome involves the action of ESCRTs. These molecules are also involved in the secretion of exosomes outside the cell4. TDEs often contain tumor-specific antigens expressed in the parental tumor cells; some examples are melan-A, Silv, carcinoembryonic antigen (CEA), and mesothelin. These antigens are presumably transferred to dendritic cells (DCs) and can induce CD8 T cell-dependent antitumor effects in both mice and humans5. The transfer of contents in exosomes is specific to ligands or signals on the recipient cells and exosomes1.
Besides facilitating effects which promote tumor progression and invasion, there are factors within TMEs which can inhibit antitumor immunity by promoting immunosuppression. The TME of TDEs are different from other local cellular environments in that the vasculature is poorly organized, creating a hypoxic environment which significantly hinders the transfer of many types of molecules 1. In inflammation and cancer, there are common molecules and signaling pathways, such as nuclear factor kappa B (NFκB), signal transducer and activator of transcription 3 (STAT3), interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha (TNF-α). NFκB has been demonstrated to be related to the antitumor and pro-tumor properties of macrophages3.
Immune-regulatory activities of tumor-derived exosomes on immune cells
Natural Killer (NK) Cells
NK cell-derived exosomes contain cellular perforin and granzyme B3. TDEs were compared to NK cell-derived exosomes for their ability to neutralize these substances78. Reduced expression of MHC I is one of the mechanisms by which cancer cells used to escape from T-cell recognition. However, this is not a barrier for NK cells since they possess a large number of target recognition receptors and do not require the need for T cell receptors (TCRs/CD3)9. Fas ligand (FasL) found in the structural components of exosomes derived from cancer cells can be cytotoxic to NK cells2. The expression of PD-L1 mRNA in exosomes may outweigh the effects of anti-PD1 antibody treatment 2 as another way for the cancer to escape immunotherapy. The ligand of NKG2D is an induced self-protein which is rarely manifested in normal cells, but is over-expressed in cells that are transformed, infected, stressed, or aging. It was shown that TDEs express ligands for NKG2D and down-regulate these receptors on NK cells 1. Liu. (2006) showed that TDEs blocked IL-2-mediated perforin production of NK cells, and blocked expression of JAK3; cyclin D3 then prevented NK cells from entering the cell cycle 10. TDEs carry a complex of MIC ligand A and B that bind to NK, impairing the ability of these cells to recognize tumor cells while reducing the expression of NKG2D receptors on the surface of NK cells 11.
CD8 T-cells
According to Maybruck (2017), induction of purified CD3CD8 T cells by exosomes derived from head and neck squamous cell carcinoma (HNSCC) elicited the suppressor phenotype (SP), causing the loss of CD27/CD28 expression and attenuation of interferon gamma (IFN-γ) production. SP CD3CD8 T cells are also capable of inhibiting CD3CD8 T cells outside the tumor. This inhibitory phenotype is driven not only by the protein content but also by the mRNA content of the exosomes12. Liu (2020) demonstrated that exosomes isolated from gastric cancer cell lines created an immunosuppressive TME by increasing numbers of effector memory CD4 T cells and myeloid-derived suppressor cells (MDSCs), decreasing CD8 T cell function ( apoptosis) and NK frequency/function, and promoting gastric cancer lung metastasis. Simultaneously, gastric cancer cell lines also increased their secretion of IL-2, IL-6, IL-10, and IFN-γ; among them, IL-10 levels increased the greatest (up to 12-19-fold). The IL-10 increase was accompanied by expression of immune suppressive genes, such as FOXP3, in TDEs (from the tumor derived CD8 T cells)6. Exosomal FasL and TNF-related apoptosis-inducing ligand (TRAIL)- bound in TDEs- can also induce apoptosis of activated CD8 T cells1, 2. The TME has an important role in the polarization of CD8 T lymphocyte sub-population. The majority of APCs in TME and tertiary lymphoids are dysfunctional and produce altered cytokine profiles and co-stimulatory molecules, which greatly influence the lineage commitment of CD8 T cells13. The engagement between MHC I (of TDEs) and CD8 receptor (of T cells) leads to apoptosis of T cells via the activation of the Fas/FasL signaling pathway2.
CD4+ T-cells
Exosomes derived from gastric cancer showed a decreased frequency of naïve T cells but increased frequency of effective memory T cells (CD62CD44)6. Studies have shown that TDEs promote Treg and MDSC expansion2. TEDs can indirectly activate CD4 T cells and inhibit the activity of CD8 T cells through cancer-associated fibroblasts (CAFs)14. Exosomes derived from nasopharyngeal carcinoma inhibit Th1 and Th17 differentiation and induce Treg differentiation 15. According to Laurent . (2016), TDEs decreased the expression of CD69 on the surface of activated CD4T cells, and increased adenosine production of Tregs in a ligand-receptor, but not internalized pattern 16. For CAR T cell-based therapy in the treatment of neuroblastoma, TDEs counteracted the effects of CD4T cells, but not CD8T cells17.
Dendritic cells (DCs)
DCs are the most professional antigen-presenting cells (APCs) of the immune system18. Exosomes from gastric cancer cell lines were taken up by NKs and macrophages much more than by CD4 and CD8 T cells, but were not taken up by DCs6. In the cases of human colorectal carcinoma and melanoma, exosomes were shown to have the ability to inhibit the differentiation of human monocyte precursors to dendritic cells or macrophages”2. CD27/CD28 expressed in T cells is a ligand of the stimulating co-receptor on APCs. Galectin plays an important role in creating an immunosuppressive environment for tumor growth, as well as for promoting the invasion and metastasis of various malignant cells19, 20. The loss of CD27/CD28 expression mediated by galectin-1 was observed in TDEs12. This activity was demonstrated to be related to the Treg activation component of the immune system20. In addition, the increase in expression of galectin-1 was directly proportional to MMP-1/2, and associated with tumor metastasis and angiogenesis21. However, in other studies, galectin-1 had the effect of inhibiting the proliferation and metabolic activity of breast cancer cells22.
Myeloid-derived suppressor cells (MDSCs)
Via tumor growth factor beta (TGF-β) and prostaglandin E2 (PGE2) from TDEs, myeloid precursor cells can be differentiated into MDSCs, which release immunosuppressive factors, such as nitric oxide (NO) and reactive oxygen species (ROS)1.
Moreover, it has been found that exosomes derived from breast cancer cells are also able to transform normal cells3. Glioma cells can transfer exosomal epidermal growth factor receptor variant III (EGFRvIII)- accompanied by oncogenic activity- to cells that do not have this receptor23. The exosomes of CAFs have been implicated in resistance to chemotherapy and stimulation of tumor proliferation24. Some of the well-known products of CAFs for inhibiting T-cell responses include IL-6, CXC-chemokine ligand 9 (CXCL9), and TGF14. Indeed, the signals from CDEs are responsible for converting normal fibroblasts and MSCs into CAFs25. The ECM organization of fibroblasts also has a strong influence on metastasis of cancer cells, and prevents the penetration of immune cells into the tumor microenvironment26.
Role of TME and TDEs in tumor rejection
Most reports have suggested that TDEs are beneficial for cancer development. However, there are several reports that indicate TDEs play a certain role in enhancing tumor response and elimination. For example, TDEs are a novel source of tumor-rejection antigens for T-cells27. CD8 T cells also produce EVs that inhibit tumor progression through the effect of reducing mesenchymal tumor stromal cells, in addition to the classical cytotoxicity of CD8+ T cells28.
Conclusion and Perspectives
TDEs are being targeted as a potential biomarker for cancer identification and next-generation cancer therapies1. Exosomal integrins reveal the metastatic sites of the primary tumor cells2. TDEs can be used to deliver cancer drugs because of their ability to home back to their origin29. Moreover, they contain antigens from the tumor which can be used to develop cancer vaccines5. The raw materials for production of such vaccines can be obtained from a variety of biological fluids and do not deteriorate when stored for long periods of time30. Furthermore, the development of drugs targeting the galectin-1 constituents of exosomes holds promise in the treatment of many types of cancer19. Elimination of exosomal PD-L1 inhibited tumor proliferation, even in an anti-PD-L1 antibody mouse model31. The application of TDEs, in general, and CDEs, in particular, is very promising. However, more research is needed to apply advanced technologies based on exosomes for cancer diagnosis, prognosis, treatment and control.
Abbreviations
APCs: antigen-presenting cells
CAFs: cancer-associated fibroblasts
CDEs: cancer cell-derived exosomes
CEA: carcinoembryonic antigen
DCs: dendritic cells
ECM: extracellular matrix
EGFR: epidermal growth factor receptor
ESCRT: endosomal sorting complexes required for transport
EVs: extracellular vesicles
FasL: Fas ligand
HNSCC: head and neck squamous cell carcinoma
IFN-g: interferon gamma
MDSCs: myeloid-derived suppressor cells
MVBs: multivesicular bodies
NFkB: nuclear factor kappa B
PGE2: prostaglandin E2
ROS: reactive oxygen species
SP: suppressor phenotype
STAT3: signal transducer and activator of transcription 3
TCRs: T cell receptors
TDEs: tumor-derived exosomes
TME: tumor microenvironment
TRAIL: TNF-related apoptosis-inducing ligand
Acknowledgments
None.
Author’s contributions
All author equally contributed in this work. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

