Breast cancer treatment by transplantations of dendritic cells and cytokine-induced killer cells: An update on clinical trials
- Stem Cell Institute, University of Science Ho Chi Minh City, Viet Nam
- Viet Nam National University Ho Chi Minh City, Ho Chi Minh City, Viet Nam
- tem Cell Institute, University of Science Ho Chi Minh City, Viet Nam
- Laboratory of Stem Cell Research and Application, University of Science Ho Chi Minh City, Viet Nam
- Laboratory of Cancer Research, University of Science Ho Chi Minh City, Viet Nam
Abstract
Breast cancer is the world's most common cancer in women and is the leading cause of their cancer-related mortality. Its early diagnosis with conventional therapies such as surgery, chemotherapy, and radiotherapy can give good results in most breast cancer patients. However, these therapies provide poor outcomes in metastatic breast cancers or late-stage breast cancer. Therefore, as another effort for breast cancer treatment, immunotherapy is now considered the fourth-line cancer treatment besides conventional therapies. In this article, we focus on breast cancer treatment by transplantation of cytokine-induced killer cells (CIKs) and dendritic cells (DCs). While CIKs are effector cells that can directly attack and kill breast cancer cells, DCs support other immune cells in including CIKs in antitumor activities. Although transplantation of CIKs or DCs alone gave limited results in breast cancer treatment, the combination of CIKs and DCs in current clinical trials demonstrated significant results. Thus, we propose that CIK-DC therapy will emerge as a new option for breast cancer treatment soon.
Introduction
Breast cancer is the world's most common cancer in women and is the leading cause of their cancer-related mortality. It can usually affect women of all ages. In the US itself, breast cancer incidence in women is up by 30%, recorded in 20191. During 1996 – 2015, about 14,222 new breast cancer cases were reported (including 13,948 women, accounting for 98%), and more than half were diagnosed with stage II while stage III and IV were about 26%2.
Early diagnosis of breast cancer combined with conventional therapy such as surgery, chemotherapy, and radiotherapy is the most common strategy. However, due to the heterogeneous nature of breast cancer and the incidence of metastasis, it remains incurable. Therefore, in the efforts against cancer, immunotherapy has emerged as the fourth line of cancer treatment besides conventional therapy. Immunotherapy harnesses the complexity of the natural immune system to fight cancer, either actively or passively; the strategies aim to boost host immunity to fight cancer again. Massive research on immunotherapy has produced many promising clinical results, including treatment with checkpoint inhibitory, cytokine, and adoptive cell therapies3, 4. Additionally, the breakthrough of using anti-PD-1 and anti-PD-L1 antibodies in treatment with metastatic, triple-negative breast cancer patients has illuminated the field of immunotherapy for breast cancer treatment5.
Adoptive cell immunotherapy offers an approach that selectively targets cancer with high efficiency and low risk of side effects6. Cell immunotherapy is a promising strategy aimed at improving the antitumor activity of the immune system. Based on the concept of harnessing the immune system, several concepts have been developed for cell-based immunotherapy approach, including adoptive cell therapy with LAK, TIL, CAR-T, NK, and CIK or cancer vaccine with DCs-based immunotherapy.
In this review, we focus on studying cytokine-induced killer cells (CIK) and dendritic cells (DCs) in breast cancer treatment. Cell immunotherapy is a promising strategy aimed at improving the antitumor activity of an immune system.
Cytokine-induced killer cells & dendritic cells for breast cancer treatments
CIKs and their cytotoxic mechanisms toward tumor cells
What are CIKs
Cytokine-induced killer cells (CIKs) are a heterogeneous population characterized by the frequency of three populations: CD3CD56 (NK-like T), CD3CD56 (T lymphocytes), and CD3CD56 (NK cells). This population is produced only by the culture of MNCs supplemented with cytokines. The first protocol for CIK production was introduced by Schmidt-Wolf . in the 1990s7.
How to produce CIKs?
CIK cells can be easily produced in conditions using MNCs from bone marrow, peripheral blood, or umbilical cord blood in combination with supplements of interferon-gamma (IFN-) and interleukin-2 (IL-2) with antibody-CD3 clone OKT3 over short-term of 2 – 3 weeks. The culture condition of CIK was modified from the LAK production protocol adding 1000 U/ml of INF- 24 hours before culture in the condition of anti-CD3 and IL-28. The addition of IFN-g significantly enhances CIK cytotoxicity compared to the LAK culture method. Indeed, IFN-g plays a role in inducing IL-12 production by activating monocytes9, 10. Furthermore, compared to LAK cells, CIK cells showed a higher expansion and prolonged antitumor effect without exogenous cytokine IL-211, 12.
The ratios of the three different cell populations inside a CIK population are different between culture and inducible protocol. Generally, a CIK population is characterized by average 70 — 80% of CD3 cells, CD3CD8 cells over 60–80% and CD3CD56 cells over 20 — 30%13. Antitumor effects of the CIK population are best seen in CD3CD56 population, a subset of CD3T lymphocytes that co-express natural killer cell protein CD5614, 15. The CD3CD56 subset is derived from CD3CD8 T lymphocytes, acquiring the terminally differentiated effector phenotype and granular structure of NK cells and higher levels of secreted antitumor cytokine IFN-, TNF-α, Granzyme B/Perforin15, 16, 17. expanded-CIK significantly increased CD3CD8 T cells and CD3CD56 NK-like T cells15, 17.

The antitumor activities of CIK population are acquired from the activities of three different cells inside (CD3+CD56-,CD3+CD56+, and CD3-CD56+). (A) The CD3+CD56+ cell population can kill the tumor cells by releasing Gramzym B/Perforin after interacting with tumor cells through surface markers. (B) The CD3-CD56+ cell population displays the antitumor activities similar to NK cells, while (C) the CD3+CD56- cell population exhibits the antitumor activities similar to T cytotoxic cells. https://doi.org/10.6084/m9.figshare.17104232.v1
Antitumor activity of CIK population
The antitumor activities of CIK population are acquired from the activities of three different cells inside. All subsets of CIK populations (CD3CD56, CD3CD56, and CD3CD56) display antitumor activity through various mechanisms (Figure 1).
CD3CD56 cell subset is capable of inducing MHC-unrestricted antitumor cytotoxicity14, 18. Indeed, CIKs also display their cytotoxic capacity in case of blocking of their receptors (CD2, CD3, CD8, CD28, CD56, very late antigen [VLA-4], T-cell receptor [TCR] αβ, MHC class I and II) by antibodies. The cytotoxic function of these populations heavily depends on engaging several activation receptors and releasing Granzyme-B/Perforin proteins from CIKs19. As a result of co-expression of NK and T cell markers, Pievani . (2011) suggested that the CD3CD56 cells acquire dual cytotoxic functions16, which stem from NK-cytotoxic functions and T-cell cytotoxic mechanisms. Antitumor activities of CIK cells require the direct interaction between CIK and tumor cells through surface markers16, 20. These interactions induce the release of granzyme B and perforin to mediate CIK-related killing function and promote IFN- and TNF-α production21. The interactions via receptors between CIKs and tumor cells are not well-understood; some recent studies suggested four main interactions between CIKs and tumor cells. The first interaction is performed by receptor leukocyte function-associated antigen-1 (LFA-1) on the CIKs with their ligands in tumor cells (ICAM-1, -2, and -3). Indeed, if the LFA-1 or ICAM-1 is blocked, the cytotoxic potential is significantly reduced15, 22, 23. The second interaction that plays an essential role in tumor recognition by CIK cells is the natural killer group 2 D (NKG2D) receptor on CIK cells and their ligands in tumor cells. NKG2D receptor is a member of the c-type lectin-activating receptor family expressed in the NK cells and NKG2D ligands: stress-inducible molecules on both solid and hematologic tumors, such as the MHC class I-related molecules A and B (MIC A/B) and members of the UL16-binding protein family (ULBP1-4) expressed in tumor cells. Interestingly, NKG2D ligands appear to express a pattern relatively restricted to malignant tumors24, 25. The expression of NKG2D receptors is involved in the high dose of IL-2 presenting in the culture medium. Besides IL-2, IL-15 also seems to be a target recognition of NKG2D26. The third interaction relates to the expression of CD56 expressed on CIKs with their ligands in tumor cells. Introna . suggested that CD56 plays a role in tumor recognition and cytotoxicity of CIK cells. Therefore, when antibodies blocked CD56 in the CIKs, the cytolysis of CIKs reduced27, 28. The fourth interaction relate to Fas ligand (FasL) highly expressed in CIKs and Fas on tumor cells8, 29. In a recent study, Meng et al. analyzed the transcriptomic atlas of CIKs and confirmed the high expression of FasL in CIKs30. The direct contact of FasL on Fas triggers Fas-dependent apoptosis mechanism in tumor cells15, 16, 19. Recently, the interactions of NKp30 and DNAM-1 expressed in CIKs with their ligands on tumor cells play a role in antitumor cytotoxicity of CIKs16.
Some recent studies revealed that CIK could perform the antibody-dependent cell cytotoxicity mediated by the expression of CD16. This observation is different between groups. Some studies suggested a subset of CD3CD56CD16 in the population of CD3CD56+ 31, 32, 33, 34, while other groups did not detect the expression of this protein in the CD3CD56 population11, 15, 35. Cappuzzello suggested that the expression of CD16 in the CIK population was donor-dependent8. In a study of 60 samples, the CD3CD56CD16 population ranged from 2.3% — 54.2% (mean 16 ± 13.3%). study and mAbs enhanced the specific lysis rate of CIK cells against EGFR- and Her-expressed cell lines. In TBNC-Patient-Derived Xenograft (PDX) models, treatment combining monoclonal antibody (mAb) and CIK significantly prolonged survival and reduced tumor volume. According to tumor section analyses, the combination also resulted in a higher infiltration of immune cells in the tumor36.
antitumor activity of CIKs
In a preclinical study, infused-allogeneic CIK cells able to locate and increase in spleen and cervical lymph nodes and remain in tumor site for up to 21 suggested prolonged antitumor effects. In regard to graft versus host disease (GVHD), models, dose up to 20.10 allogeneic CIK cells was tolerated well compared to naïve T cell infusion, which quickly developed severe acute GVHD18. CIK cells demonstrated antitumor activity toward a wide range of cancer cell lines and freshly isolated cancer cells. Furthermore, numerous studies proved CIK's ability to treat both hematologic and solid tumors13, 20, 21, 37, 38. Recently, Capellero published a preclinical study; the CIK cells from EOC patients efficiently killed patient-derived ovarian cancer cell lines (pdOVC), with no difference between autologous and allogeneic targets39. Also, the study indicated that CIK cells also efficiently killed chemotherapy-survived pdOVC; the killing ability was superior due to the high expression of stress ligand in tumor cells after being treated with carboplatin. In models, CIK infusion resulted in high necrotic areas and a high rate of CIK infiltration. In a study with breast cancer cell line MCF-7, CIK strongly inhibits proliferation of both radio-resistant and normal MCF-7 cells17, 40.
Clinically, in 2020, Ying Zhang and Schmidt-Wolf published an updated international registry over the past ten years of CIK immunotherapy13. A total of 106 clinical trials was registered through IRCC; 4,889 patients with more than 30 types of cancers received CIK treatment along with/without conventional therapy. Treatment with CIK-based therapy significantly improves mPFS and mOS of patients. Patients’ immune system was significantly altered: CD3CD56, CD3CD8, CD4/CD8 population ratio were elevated while T-reg population CD4CD25FoxP3 was decreased. Also, the level of Th-1 associated cytokine was increased after CIK treatment. In terms of safety, CIK treatment-related side effects were mostly grade I and II such as fever, chills, fatigue, headache, and skin rash. The incidence of grade III–IV toxicities was rare in the CIK treatment group. Infusion of allogeneic CIK was related to acute and chronic GVHD; however, patients showed good –tolerance the immunosuppressive regimen. The CIK treatment reported a higher Karnofsky score (KPS), better appetite, improved sleep, weight gain, and pain relief.
DCs and their cytotoxic mechanisms toward tumor cells
What dendritic cells are?
Dendritic cells (DCs) are known as professional antigen-presenting cells (APCs). The DCs’ ability to present antigen attracts attention as carriers for cancer vaccine approaches41. In the body, DCs are activated and matured in response to the environmental stimulator. The activation of DCs further mediates T cell activation through the engagement of MHC-class I/II and co-stimulation with cytokines. The DC-based vaccinations inhibit tumor growth by altering host lymphocyte composition. expanded TAA-loaded DCs have been widely approached in a clinical study for targeting tumor and boosting specific-targeting immune response. Over the past two decades, DC-based therapy represents a feasible approach to elicit antitumor immunity while remaining safe and well-tolerated in patients.
How to produce DCs?
Current approaches of DC-based immunotherapy include the use of isolated CD14 monocytes or CD34 HPC from blood or bone marrow42. Several protocols have been developed using unstimulated DCs, ex-vivo matured DCs or cell-lysate/TAA-pulsed DCs, in-situ DC vaccination, and DC-derived exosomes43.
The first generation used tumor antigen-loaded immature DCs and achieved poor clinical response with only 3.3% tumor regression. The second-generation DC vaccines used matured monocyte-derived DCs, and the treatment reached 8 — 15% objective response rates with the median OS increasing by ~20% in some studies44.
The roles of DCs in anti-tumors
Unlike CIKs — effector cells that can directly attack cancer cells and kill them — DCs are antigen-presenting cells so that they indirectly strengthen the antitumor process of the immune system. However, they play an essential role in immune response in cancer treatment. Indeed, cancer cells usually escape from the immune surveillance in cancer patients, especially the sub-population of cancer stem cells inside. The effector T cells inside these patients cannot recognize cancer to kill them. DCs, in this case, will activate the T cells (both CD4 and CD8 cells). They can give some essential boost to immune responses in antitumor activity:
DCs enable CD4 T cells to activate B and CD8 cells. This process is based on the interaction between DCs and CD4 T cells through CD40. The CD40 in DCs will interact with the CD40 ligand in T cells leading to DC activation. In the activated state, DCs can prime T cells and up-regulate the expression of some co-stimulatory molecules and produce IL-12. Then, IL-12 causes polarization in naïve CD4 cells toward Th1 cells or Th2 cells. Th1 cells and Th2 cells will promote CD8 cells and B cells through some cytokines (IL-2, IL-4, IL-5, IL-13, and IFN-g).
DCs also cross-talk with NKs and play a pivotal role in the innate immune response against cancer45. DCs interact with NKs via CXCR3 in the draining lymph nodes in a "touch and go" mode lasting from 300s to 4h46. As a result, DCs will produce IL-12, IL-18, IL-27, type I IFNs, and IL-15, PGE2. These cytokines directly affect NK cells, triggering NK cell proliferation and activating NK cells. The activated NK cells leave the lymph node, infiltrate tumors, and attack cancer cells in the tumors.
antitumor activity of DCs
Clinical trials of DC vaccination showed promising results. In the role of APC, DCs are used to present tumor antigens to other immune cells. Therefore, both tumor-specific antigens and tumor-associated antigens are used in DC vaccinations. These antigens can be peptides/proteins, mRNA, or tumor lysates47. Thus, DC vaccination appears a safe and feasible strategy; furthermore, the vaccination combines with antigen-specific CTL activity and positive natural killer response in > 50% cancer patients48, 49.
In 2010, sipuleucel-T, the first cellular-based immunotherapy, was approved by USA FDA for the treatment of prostate cancer patients. The intervention was activated DCs by recombinant fusion protein PA2024, the fusion of GM-CSF with prostate antigen, which can be classified as the intersection between first and second generation of DC vaccines. The randomized clinical trial was conducted on 512 patients; sipuleucel-t treatment increased median survival by 4.1 months compared to the placebo group (25.8 months vs. 21.7 months, respectively); however, the treatment failed to achieve better disease progression50. Various kinds of cancer also were clinically treated by DCs such as glioblastoma51, 52, acute myeloid leukemia53, 54, breast cancer55, metastatic colorectal cancer56, prostate cancer57, mesothelioma58, lung cancer59, hepatocellular carcinoma60, pancreatic cancer61, advanced melanoma62, non-small cell lung cancer63, bone and soft tissue sarcoma64, and myeloma65, 66.
Collaborative mechanisms of CIKs and DCs in antitumor cells
Since DC therapy aims to improve host adaptive immune responses, different strategies have been developed harnessing the immune-stimulation effects of DC with effector cell-based immunotherapy . Stimulation activity of DCs is through the ability to capture, processing and presenting a tumor-associated antigen (TAAs), which induces specific antitumor responses. The intervention combining DCs and T cells ex vivo resulted in a lower risk of relapse and metastasis, lower level of T-reg, and increased Th1 polarization in breast cancer patients67.
In recent years, several studies have reported that the synergistic antitumor effect of CIK blends with DCs13, 21, 68. The strategy provides the ability to target cancer cells in an MHC-independent manner through CIK cells, while DCs mount an immune response through an MHC-restriction mechanism. Co-culture of CIK and DCs significantly improved the antitumor effect by increasing cytokine IL-12 and IFN-g production; the interaction is a TCR-independent mechanism29, 69, 70. Recent studies have proved that DC enhances CIK through cell-cell contact in an MHC-independent manner but by CD40L/CD40; inhibiting CD40L/CD40 interaction abrogates these changes70, 71. Interaction of DCs and CIK thus alters the expression of several membrane proteins, including upregulation of membrane protein CD28 and CD40L on CIK, which are co-stimulatory signals promoting immune activation. Further, an increase in proliferation and CIK phenotype (including CD3, CD8, and CD3CD56 population) has been observed after co-culture with DCs72. The concomitant of Treg in CIK population also decreases in both cell and mRNA levels after co-culture with DCs72, 73. In models, DC plus CIK revealed a superior antitumor effect compared to single therapy74. The treatment altered host immunity, altering host immune-system composition and augmenting immune antitumor response by enhancing CTL and NK-cell function. The ratio of CD4/CD8 significantly increased after DC/CIK treatment, represented the immunomodulatory effect, and improved immune-surveillance of DC-CIK to the host immune system75, 76. Also, the level of Th1-associated cytokine was elevated, including IL-2, IFN-g, IL-12. In reverse, the treatment resulted in a lower proportion of immunosuppressive factors, T-reg cell, cytokine IL-10, and TGF-β77, 78.
DCs plus CIKs have been proved as a promising immunotherapy approach in treatment of advanced solid tumors. In a ten-year review, 37 of 85 studies were conducted with DC-CIK treatment for lung cancer, hepatocellular carcinoma, pancreatic cancer, colorectal cancer, renal cell carcinoma, and breast cancer13. DC-CIK treatment shows significantly enhanced response rate in patients, better clinical benefit rate, and higher median overall survival compared to conventional treatment group.
Clinical trials of CIK and DC-CIK cells on breast cancer patients
|
Year (Ref) |
Study type |
Disease |
Patients (treatment) |
Pre-treatment |
Intervention |
Immunotherapy clinical response |
|---|---|---|---|---|---|---|
|
2014 |
Retrospective |
TNBC (stage I-III) |
90 (45) |
Surgery, adjuvant chemotherapy with or without radiation |
autologous CIK cells 8.7 x 109 – 1.2 x 1010 cells/cycle (4 - 52 cycles) |
1-, 2-, 3-, and 4-year DFS rate: 97.7%, 90.1%, 83.4%, 75.2%. 1-, 2-, 3-, and 4-year OS rate: 100.0%, 100.0%, 96.7%, 92.4%, |
|
2018 |
Retrospective |
TNBC |
340 (77) |
Surgery, chemotherapy |
autologous CIK cells 5 - 7.7 x 109 cells/cycle (1 - 19 cycles) |
5-years OS rate 94.3% 5-year DFS rate 77.9% PD: 16/77 cases (20.8%) Death: 4/77 cases (5.2%) |
|
2019 |
Retrospective |
BC stage I-III |
310 |
Surgery with chemotherapy or radiotherapy or endocrino-therapy |
autologous CIK 8.7 - 12 x 109 cells/cycle (at least 4 cycles) |
5-year OS rate: 85.7% 5-year RFS rate: 80.8% |
|
2019 |
Retrospective |
TBNC |
294 |
Surgery and chemotherapy |
autologous CIK > 5 x 109 cells/cycle ( 1 - 26 cycles) |
1-, 3-, and 5-year DFS rate: 99.3%, 91.8%, 99.1% 1-, 3-, and 5-year OS rate 99.3%, 96.6%, 93.4% |
|
2015 |
Clinical trial |
Metastatic breast cancer |
20 |
Chemotherapy |
autologous DC and CIK 1 x 109 CIK with 1 x 107 DCs per cycle (8 cycles) |
CR: 3/20 cases PR: 12/20 cases SD: 2/20 cases PD: 3/20 cases |
|
2013 |
Randomized Controlled Trial |
Metastatic breast cancer |
166 (87) |
Chemotherapy |
HDC with autologous DC/CIK (3 cycles) |
mOS: 33.1 months 3-year OS rate: 20.7% (18/87 patients). 4-year OS rate: 9.2% (8/87 cases) |
|
2015 |
Clinical trial |
Advanced cancer stage IV |
12 cases with breast cancer |
Surgery or chemotherapy or radiation |
autologous DC/CIK 5.7 (6 cycles) |
DCR: 25% (3/12 cases) |
|
2016 |
Clinical trial |
TNBC |
23 |
Chemotherapy |
autologous DC/CIK (3 cycles) |
PR: 3/23 SD: 56.5% (13/23) PD: 30.4% (7/23) ORR: 13% DCR: 69.6% mPFS was 13.5 months |
|
2017 |
Retrospective |
Stage IV brast cancer |
368 (188) |
Chemotherapy |
autologous DC/CIK 6 - 10 × 109 cells/infusion (4 infusions/cycle, > 3 cycles) |
5-year DFS rate: 42% 5-year OS rate: 44% |
Registered clinical trials on ClinicalTrials.gov database
|
Year |
Identifier |
Phase |
Disease |
Intervention |
Status |
|---|---|---|---|---|---|
|
2010 |
NCT01232062 |
not showed |
Breast Neoplasms Neoplasm Metastasis |
High dose chemotherapy with DC-CIK |
Completed |
|
2011 |
NCT01395056 |
not showed |
Breast Neoplasms Neoplasm Metastasis |
Cyclophosphamide combined thiotepa and carboplatin chemotherapy combined with DC-CIK immunotherapy |
Completed |
|
2015 |
NCT02491697 |
Phase 2 |
Breast cancer |
DC-CIK immunotherapy with capecitabine |
Active, not recruiting |
|
2015 |
NCT02539017 |
Phase 2 |
Triple Negative Breast Neoplasms |
DC-CIK combined with chemotherapy |
Withdrawn |
|
2015 |
NCT02450357 |
not showed |
Neoplastic Cells, Circulating |
DC-CIK immunotherapy |
Completed |
|
2016 |
NCT02886897 |
Phase 1 Phase 2 |
Breast Cancer |
DC-CIK and anti-PD-1 antibody |
Unknown |
|
2018 |
NCT03524261 |
Phase 2 |
Advanced breast cancer |
Activated CIK and CD3-MUC1 Bispecific Antibody |
Withdrawn |
|
2020 |
NCT04282044 |
Phase 1 |
Triple Negative Breast Cancer (advanced solid tumors) |
CRX100 suspension (autologous CIK) |
Recruiting |
|
2020 |
NCT04476641 |
Phase 2 |
Breast cancer |
DC-CIK immunotherapy |
Recruiting |
Transplantation of CIKs and DCs to treat breast cancer
Transplantation of CIK cells in the treatment of breast cancer
The clinical approach of CIK-based adoptive cell therapy has been growing vigorously in recent years (
In 2014, a retrospective study was published by Ke Pan .73; the study included 90 patients with TNBC status; 45 of them received adjuvant CIK immunotherapy (8.7 x 10 – 1.2 x 10 cells/infusion) after completed chemotherapy without radiation therapy post-mastectomy. Following the CIK treatment, TNBC patients experienced better DFS and OS than conventional treatment, using The Kaplan–Meier survival analysis method (P = 0.0382 and P = 0.0046, respectively). The rate of 1-, 2-, 3-, and 4-year DFS was higher in the CIK treatment group (CIK-group: 97.7%, 90.1%, 83.4%, and 75.2%, respectively; control-group: 88.9%, 64.4%, 62.1%, and 56.4%, respectively). The rate of 1-, 2-, 3-, and 4-year OS was higher in CIK treatment group (CIK-group: 100.0%, 100.0%, 96.7%, and 92.4%, respectively; control-group: 95.6%, 88.6%, 76.3%, and 72.7%, respectively). In further analysis of prognosis in TNBC patients using Cox proportional hazards regression analyses, CIK treatment and disease status were significantly associated with favorable DFS and OS results. According to the Kaplan-Meier analysis result, CIK treatment significantly enhanced OS and DFS advanced-stage group. In contrast, the early-stage TNBC showed no significant difference in response to two treatment types73, 87. In 2019, a retrospective on 294 TNBC patients showed that CIK treatment significantly enhanced 1-, 3-, and 5-year DFS (P = 0.047) and OS rate (P = 0.007) compared to the control group (adjuvant chemotherapy w/o radiation)81. Furthermore, the data showed that higher CIK infusion was correlated with a better antitumor effect; more than six cycles of CIK treatment significantly improved DFS (P = 0.02) and OS (P = 0.04). A study on 77 CIK-treated patients similarly concluded that higher cycles (> 6) are associated with better prognosis (p = 0.002 in DFS, p = 0.024 in OS) and decreased risk of death87. CIK treatment lowered the incidence of metastasis, 16/147 patients in the CIK group compared to 29/147 patients in the control. In the univariate and multivariate analysis, CIK treatment influenced DFS and OS in patients; adjuvant CIK treatment was an independent prognostic factor for both DFS (HR = 0.520, 95% CI:0.271 – 0.998, P = 0.049) and OS (HR = 0.414, 95% CI:0.190 – 0.903, P = 0.027) in multivariate analysis. In a study of 310 postoperative breast cancer patients, patients were selected via random table method for the control and the CIK treatment group. The 5-year recurrence-free survival (RFS) rate and the 5-year OS rate were higher in the CIK treatment group than the control (17). In sub-group analysis according to disease type, patients with ER/PR and HER2 significantly benefited from CIK treatment, and significantly prolonged OS was reported. TNBC patients and ER/PR/HER2 patients also showed improved prognosis factors; however, those groups were not statistically different. The study found that PD-L1 positive patients experienced better CIK treatment response than PD-L1 negative patients did; significantly higher 5-year RFS (87.6% versus 76.4%, P = 0.048) and 5-year OS (95.2% versus 77.1%, P = 0.048%) was reported. This effect was reversed in the control treatment group; PD-L1 was correlated with worsened clinical outcomes. Further, negative PD-L1 patients in two cohorts did not statistically differ in RFS and OS, thus suggesting the use of PD-L1 as a biomarker for the adoptive immunotherapy approach for breast cancer patients.
Side effect in these retrospective studies was reported mostly as spontaneous fever; no intolerable or severe side effect was recorded following CIK treatment. Furthermore, no statistical difference was observed in the incidence of adverse effects between the two treatment groups.
Transplantation of DCs in treatment of breast cancer
In breast cancer treatment, HER-2/neu pulsed DC induced the expression of co-stimulator CD28 on CD8 T cells in HER-2/neuDCIS patients88. Additionally, the treatment increased levels of Th1-cytokine IFN-g and induced HER-2/neu-specific CD8 T cells with a lower level of inhibitory B7 ligand CTLA-4. A high disease-free survival rate and prolonged median disease-free survival were achieved in the vaccinated group, compared to control treatment89. P53-pulsed DC vaccinations showed p53-specific T cell response in advanced breast cancer patients90. Patients experienced prolonged survival and temporary regression of metastasis while no toxicity was observed during DC administration. ELISpot analyses analyzed the specific T cell response; some patients experienced stable disease and lymph node regression. Overall, the clinical efficiency of DC immunotherapy remains below expectations. Poor clinical outcomes result from several factors, including lack of appropriate target antigens, downregulation of TAA and MHC molecules in tumor cells, poor homing ability of adoptive transferred-DC to lymph node, and rate of inducing target-specific CTL and immune-suppressive tumor microenvironment42. However, DC vaccination is a safe approach, thus facilitating further modifications and research to improve the clinical results.
DC-CIK cell transplantation in treatment with breast cancer
Preclinical studies have proved the superior antitumor effects of DC-combined CIK70, 74, 91, 92. DC-CIK combination has been widely used in clinical trials besides chemotherapy for cancer treatment75, 93, 94, 95, 96. Co-culturing DC and CIK leads to greater CIK anti-cancer effect against cancer cell. In clinical trials, the DC-CIK combination also showed that the clinical response outweighs conventional therapy. In five accessed clinical studies on DC and CIK application, a total of 589 breast cancer patients were enrolled, including 330 patients under DC-CIK treatment. In 2013, Ren J investigated the effect of high-dose chemotherapy and DC-CIK compared to standard dose chemotherapy for metastatic breast cancer treatment in 166 patients83. The intervention was two cycles of 120 mg/m docetaxel plus 175 mg/m thiotepa in combination with DC-CIK; the addition of carboplatin was optional. The trial group achieved a significantly higher objective response rate compared to SDC treatment, 25.9% versus 10.1%, respectively (P = 0.009). In summary, 2 CR cases (2.4%), 20 PR (23.5%) cases, and 42 SD (49.4%) cases were reported in the HDC+DC-CIK treatment group. The median-OS were double that of the control group, 33.1 months in the experiment group and 15.2 months in the control group (P < 0.001). The median-PFS also significantly improved in the experiment group, with an average of 10.2 months vs. 3.7 months (P < 0.001). In the Cox regression model, HDC plus DC-CIK treatment for HER-2 positive patients with less than three metastasis sites were correlated with better OS and PFS prognosis. In the following clinical study in 2016, 23 metastatic pre-treated TNBC patients received a combination of cyclophosphamide, thiotepa, carboplatin, and DC-CIK immunotherapy. The study reported a 13.0% objective response rate (3 PR cases) and a 69.6% disease control rate (3 PR cases, 13 SD cases). The median-FPS reached 13.5 months (95% CI, 10.1 – 16.9 months), and the median OS was 15.2 months (95% CI, 12.5 – 18.1 months)85. In 2017, Lin M . published a 10-year follow-up study from 2003–2013. About 368 staged-IV breast cancer patients were recruited, and 188 patients were treated with one cycle of low-dose chemotherapy (Carmofure) and at least three cycles of four DC-CIK infusions86. One infusion regimen included 6 – 9.10 DC-CIK cells in 250 ml saline plus 1500 U/ml IL-2 and 1% human albumin intravenously. Lymphocyte count and function were tested after DC-CIK treatment from chemotherapy treatment. Th1-type cytokine was elevated upon DC-CIK treatment, including IL-2, TNF-β, and INF-. DC-CIK treatment significantly improved OS and DFS compared to the control group. The 5-year DFS was 42% in the experiment group, while that of control group was 30% (P < 0.01). The 5-year OS was 44% in the experimental group versus 29% in the control group (P < 0.01). Additionally, DC-CIK treatment independently lowered the risk of disease progression (OR = 0.09, 95% CI 0.02 – 0.42, P < 0.01) and risk of death (OR = 0.05, 95% CI 0.01 – 0.37, p < 0.01), according to multivariate Cox proportional regression analysis. DC-CIK treatment represented a feasible cancer treatment strategy with minimal side effects. The most common side effects were related to the chemotherapy. No lethal adverse effects were reported following DC-CIK treatment. Patients have received at least one infusion of DC-CIK; no dose modification or disruption was reported. The most common side effect was fever.
In 2014, a meta-analysis study about DCs, CIKs, and the combination of DC-CIK treatment for breast cancer patients was published by Wang .97. The meta-analysis study was conducted from 27 clinical trials with 633 enrolled breast cancer patients and compared DCs and CIKs treatment versus non-DC/CIK treatment. According to the analyzed result, the 1-year survival rate of patients in the group was significantly improved (P < 0.0001) for DC/CIK treatment group. Higher rates of 2- and 3-year survival were also reported following DC-CIK treatment; however no significant statistical difference was noted between the two groups (2-year survival: 83% versus 76%, P = 0.07; 3-year survival: 64% versus 48%, P = 0.07). The Karnofsky Performance Status Scale (KPS) results showed that breast cancer patients significantly improved from DC-CIK treatment compared to non-DC-CIK therapy (OR: 12.40, 95% CI = 6.61-18.19, P < 0.0001). A higher clinical benefit rate was recorded in DC-CIK group; however, the data was not statistically different. Additionally, the study analyzed host immune response to DC-CIK therapy. Significantly increased proportion of CD3, CD4, CD16, CD4CD8, CD3CD56 immune cell subsets (P < 0.00001) and enhancement in T cell immunity function (AG-NOR: OR = 0.68, P < 0.0001) were observed after DC-CIK treatment. Several antitumor response cytokines were elevated following DC-CIK treatment, including IL-2, IL-6, IL-12, IFN-, and TNF-α (P < 0.00001). Moreover, the level of serum cancer markers was significantly decreased after DC-CIK treatment. Later, Hu .95 published a meta-analysis to compare the efficacy and safety of DC-CIK therapy versus conventional chemotherapy for breast cancer treatment. The study was conducted based on 11 randomized clinical trials with 941 breast cancer patients (including 386 cases who experienced CIK or DC-CIK, 361 cases with conventional chemotherapy only), with no statistical difference between the two groups of patients. Most studies (9/11 studies) reported CR and PR; the difference was significant between the CIK-DC treatment group and the conventional treatment group (CR: RR = 1.54, 95% CI: 1.09-2.19; PR: RR = 1.33, 95% CI: 1.11 – 1.59). In the meta-analysis, ORR was reported in 10 studies, significantly different between DC-CIK and conventional groups (RR = 1.37, 95% CI: 1.20 – 1.57). The incidence of side effects was not significant between DC-CIK treatment and non-DC-CIK and conventional treatment groups in both meta-analyses. Side effects included fever, leucocyte decrease, gastrointestinal adverse effects (OR: 0.72, 95% CI: 0.36 – 1.45, P = 0.36)97 and leukopenia, thrombocytopenia, hair loss, nausea/vomiting, hepatic complications, and neurologic complications95.
Future perspectives
Current clinical trial data demonstrated that DC-CIK is a promising approach for breast cancer treatment21. With chemotherapy's success in improving clinical response, the combination of DC-CIK prolongs survival in breast cancer patients. In a recent study by Ren J . in 2013, DC-CIK combined with HDC was used as first-line treatment for metastatic breast cancer patients. Patients experienced delayed disease relapse and longer survival time83. Success in the clinical trials attracted research on CIK and DC; several strategies have been evaluated and . DC-CIK cells efficiently targeted cancer stem cells; autologous CIK cells inhibited tumor growth in PDX models91, 92, 98, 99, 100. Recent studies have focused on modified CIK cells with chimeric antigen receptors to enhance CIK cytotoxic function and cancer-targeting ability8, 101, 102. Ren . incorporated CIK cells with anti-EGFR chimeric antigen receptor (CAR); the CAR-CIKs showed superior antitumor target against EGFR-positive tumor cells103. The combination of CAR and CIK further enhanced the secretion of IL-2 and IFN-gamma by CIK cells. Besides aiming to modify CIK cells, the combination of CIK cells with commercial immunotherapy drugs is also under investigation. The combination of mAbs with CIK cells showed promising results in preclinical studies34, 36, 104. It improved cytotoxicity of CIK cells via ADCC and increased infiltrated CIK cells in tumor specimens. The prospective study by Zhou showed that PD-L1 expression in TNBC patients correlated with better response to CIK treatment, thus suggesting the combination of PD-L1/PD-1 immunotherapy treatment with CIK cells17.

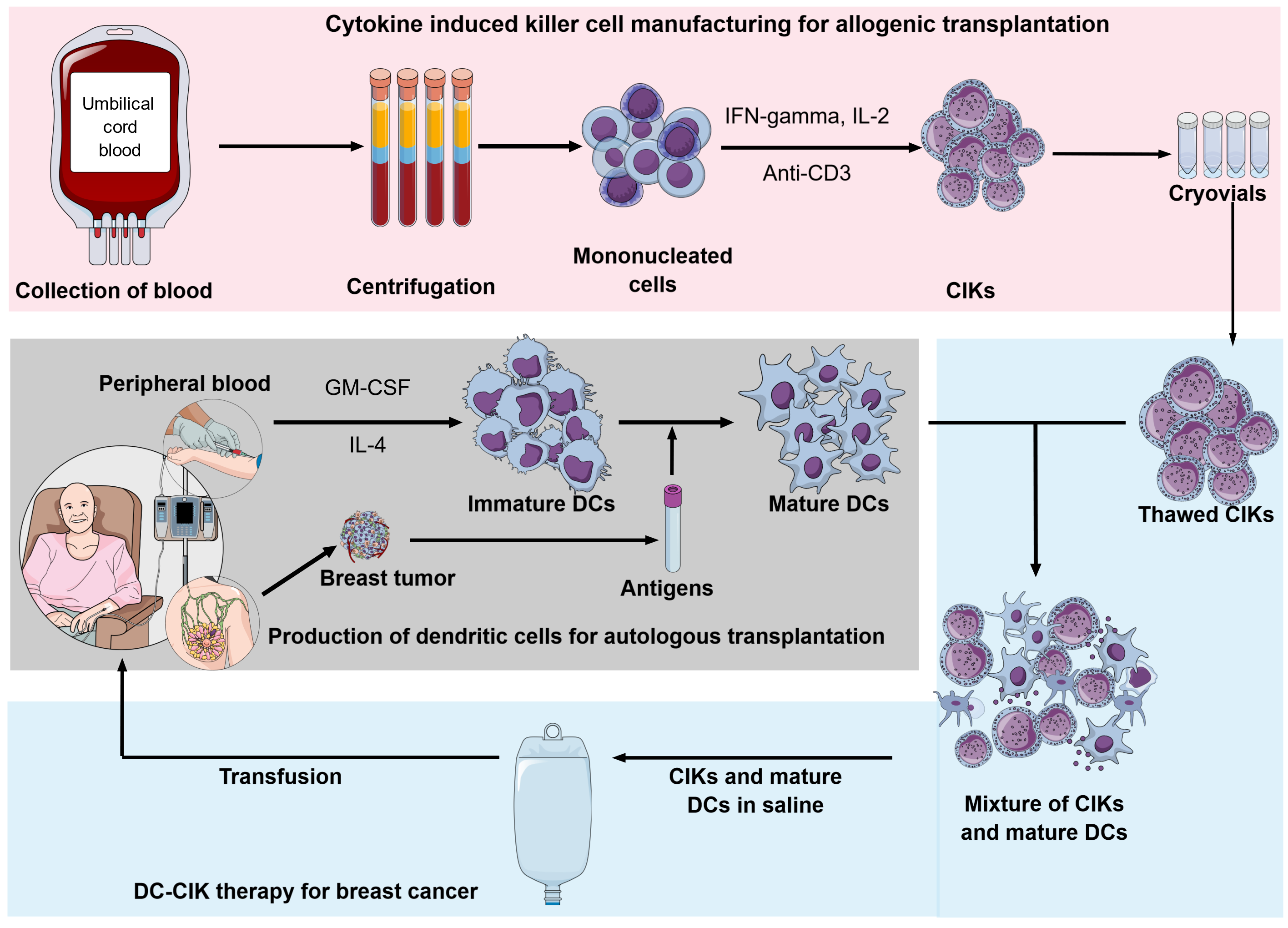
New approach of DC-CIK therapy for breast cancer using autologous dendritic cells and allogenic CIKs. Allogenic CIKs are produced from umbilical cord blood and storaged in freezer until usage. Mature DCs are produced from mononucleated cells derived peripheral blood induced with cytokine (GMCSF, IL-4) and primed with antigens from breast tumors. Thawed CIKs and mature DCs are mixed and incubated before they are used to treating the breast cancer. https://doi.org/10.6084/m9.figshare.17104241.v1
In addition to the traditional autologous approach, among several cell-based immunotherapies, CIK cells are suggested as potential allogeneic cellular immunotherapy capable of approaching an "off-the-shelf" strategy (Figure 2). The preclinical trial had demonstrated low GVHD ability of CIK cells: allograft of CIK cells associated with graft-versus-tumor (GvT) showed minimal graft-versus-host-disease (GvHD) side effect14, 18, 19, 105. The clinical trials showed that allogeneic CIKs after hematopoietic stem cell transplantation (HSCT) showed a low incidence of GvHD in recipients while inducing antitumor response106, 107, 108. In 2012, Linn reported a clinical trial phase I/II with allogeneic-HSCT relapsed patients; in five patients who developed immune responses attributed to CIK cell infusion, the risk of acute-GvHD was low (3/16 patients) and easily controlled107. In the combination of allogeneic CIK with donor lymphocyte infusion (DLI), the incidence of a GVHD was mostly associated with DLI (8 of 12 cases, total 16%)109.
Moreover, umbilical cord blood is an abundant and available source of precursor cells for CIK; more cord blood cells exert low immunogenicity110, 111. The UCB-CIK cells showed greater proliferation capacity, lower immunogenicity, lower expression of inhibitory receptor PD-1, and less susceptibility to chemotherapy than PB-CIK cells do. Additionally, the UCB-CIK cells showed higher production of IFN- and IL-2 compared to PB-CIK cells. The antitumor effect was also higher in the UCB-CIK treatment group both and 112. The clinical study further demonstrated the antitumor potential of UCB-CIK with minimal toxicities113, 114.
Further, large-scale production of GMP-grade CIK is under vigorous study; Castiglia S et al. and Palmerini P . suggested the significant impact of culture systems on CIK cell quality115, 116. Serum-free conditions were studied to abrogate the in-consistent quality of human serum and human pool plasma. A recent study demonstrated the uniformity of cryopreserved-CIK cells for up to one year117. Cryopreserved-CIK and cryopreserved PBMC derived-CIK maintained their cytotoxic function toward cancer cells, however, they were lower than freshly-cultured CIK cells117, 118.
Conclusion
In recent years, immunotherapies for breast cancer treatment have been developed vigorously. The success of DC and CIK in both preclinical and clinical studies demonstrated their position in first-line treatment. Besides, it is worth noting that DC and CIK cells are easily expanded in conditions in GMP with a high expansion rate compared to other adoptive cell therapies. Further, the use of CIK in a clinical trial is IL-2 independent, thus reducing the cytotoxicity of exogenous IL-2. Therefore, the treatment represents a promising approach with safety, tolerability, and minimal toxicity. In breast cancer treatment, the use of DC, CIK, or DC-CIK significantly prolonged the survival of patients, improved quality of life, and increased the patient's immunity function. However, the database was limited to China, the dosage of DC-CIK remained heterogeneous, and CIK cells’ function depended on donor quality. The clinical reports also showed inconsistent format and bias results. Therefore, it is essential to optimize the procedure of DC-CIK therapy to create standard criteria for evaluating DC-CIK. Furthermore, the DC-CIK therapy should be assessed in multi-centered studies on a larger scale and uniform patient disease status.
Abbreviations
CAR-T: Chimeric antigen receptor T cell
CIK: Cytokine induced killer cell
DFS: Disease-free survival
DLI: Donor lymphocyte infusion
GM-CSF: Granulocyte-macrophage colony-stimulatin factor
GvHD: Graft-versus-host-disease
GvT: graft-versus-tumor
HDC: High-dose chemotherapy
HSCT: Hematopoietic stem cell transplantation
ICAM: Intercellular cell adhesion molecule
IFN: Interferon
IL: Interleukine
LAK: Lymphokine-activated killer cell
MHC: Major histocompatibility complex
MIC A/B: MHC class I-related molecules A and B
NK: Natural killer cell
NKG2D: Natural killer group 2 D
OS: Overall survival
PB-CIK: Peripheral blood derived cytokine induced killer cell
PBMC: Peripheral blood mononucleated cell
RFS: Recurrence-free survival
TAAs: Tumor-associated antigen
TBNC: Triple-negative breast cancer
TCR: T-cell receptor
Th1: T helper cell 1
TIL: Tumor-infiltrating lymphocyte
T-reg: Regulatory T cell
UCB-CIK: Umbilical cord blood derived cytokine induced killer cell
VLA-4: Very late antigen 4
Acknowledgments
None.
Author’s contributions
All authors equally contributed in this work. All authors read and approved the final version of the manuscript for submission.
Funding
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number C2020-18-26.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

