Morin: a promising nutraceutical therapy for modulation of the NF-κB/NOX-2/IL-6/HO-1 signaling pathways in paracetamol-induced liver toxicity
- Medical Labs Department, Faculty of Applied Medical Sciences, October 6 University, Sixth of October City, Egypt
- Department of Molecular Biology, Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Menoufia, Egypt
- Biochemistry Department, Faculty of Applied Medical Sciences, October 6 University, Sixth of October City, Egypt
Abstract
Introduction: Paracetamol overdose potentially causes liver injury. This study aimed to evaluate the impact of morin against paracetamol overdose-induced hepatotoxicity.
Methods: Thirty albino rats weighing 185 ± 5 g were randomly selected from five groups: group I: orally administered with 1% Tween 80; group II: administered with 1 g paracetamol; group III: administered with 1 g paracetamol and 50 mg morin; group IV: administered with 100 mg paracetamol and morin; and group V: administered with 100 mg paracetamol and silymarin, with all treatments administered for 14 days.
Results: Morin and silymarin significantly protected the rats against induced alterations in the plasma total cholesterol, triacylglycerol, and HDL-C levels as well as the liver ALT, AST, ALP, LDH, protein thiol, GSH, SOD, CAT, MDA, and tumor necrosis factor-alpha levels. Furthermore, morin significantly inhibited the expression of NF-κB, NADPH oxidase-2, and interleukin-6 and induction of heme oxygenase-1 compared with paracetamol. The histological results indicated that morin protected the liver tissues against the toxic effect of paracetamol.
Conclusion: Morin significantly depletes the side effects of paracetamol and protects the liver tissue from the resulting free radicals.
Introduction
Paracetamol is often used to treat mild aches and pains, including headaches. In high doses, it is specifically toxic to the liver and generally causes liver necrosis, renal injury, or death in humans and laboratory animals1, 2 . Paracetamol is bio-transformed in the liver into the N-acetyl-para-benzoquinone imine (NAPQI) metabolite, which causes liver damage3.
The resulting free radical scavengers have a wide spectrum of biological activity4, 5, 6, 7. In the scientific literature, many hepatoprotective biologically active compounds exist, including natural and synthetic compounds8, 9, 10, 11, 12.
Several antioxidant-rich alternatives decrease the liver injury caused by paracetamol in animal models13. A variety of plant extracts containing saponins, polyphenols, and flavonoids have been utilized to treat a wide range of disorders10, 11, 12, 13. Nutraceuticals and herbal medications are important owing to their low toxicity, availability, and less side effects compared with synthetic drugs4, 5, 6, 7, 10, 11, 12, 13.
Morin is a flavanol that has been isolated from a variety of plants14, 15. It has been demonstrated to have free radical scavenging15, antioxidant16, 17, and anti-inflammatory18, 19 effects in numerous diseases, including liver20 and lung diseases20, 21, diabetes22, myocardial infarction23, and cancer24, 25, 26. The pharmacological properties of morin are mediated by modulation of various cellular cytokine signaling pathways27, 28, 29, 30, 31. Many reports have proven the biological importance of natural products, including morin, in medicine and pharmaceutical applications10, 11, 12, 13. In this study, we evaluated the pharmaceutical impact of morin on paracetamol-induced liver toxicity in rats.
Methods
Chemicals
Morin (98%) (Figure 1), paracetamol (99%), and silymarin (98%) were purchased from Sigma-Aldrich (Germany).
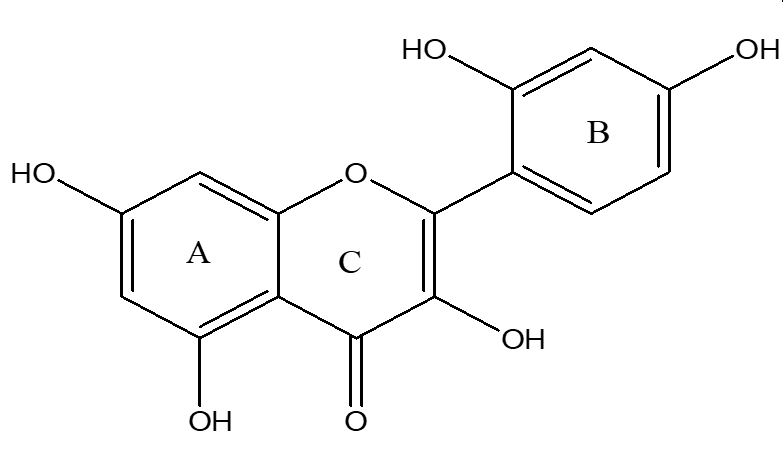
Chemical structure of morin
Animals
Adult albino rats weighing roughly 185 ± 5 g were obtained from the animal house at the National Institute of Cancer and maintained in an observational environment unders daily observation. They were housed in cages and provided free access to food and water.
Description of treatment groups
|
Groups |
Group name |
Treatment description |
|---|---|---|
|
I |
Normal 1 % tween 80 |
3 mL of 1 % tween 80, orally for 14 days |
|
II. |
Control Paracetamol |
3 mL of 1 % tween 80, orally for 14 days |
|
III |
Paracetamol + Morin |
Rats treated with Morin (50 mg/kg.b.w.) suspended in 1 % tween 80 by oral gavages for 14 days. |
|
IV |
Paracetamol + Morin |
Rats treated with Morin (100 mg/kg.b.w.) suspended in 1 % tween 80 by oral gavages for 14 days. |
|
V |
Paracetamol + Silymarin |
Rats treated with silymarin (100 mg/kg b.w.) suspended in 1 % tween 80 by oral gavages for 14 days. |
Experimental setup
The hepatoprotective activity of morin when administered for 2 weeks against paracetamol-induced hepatotoxicity was evaluated. The Animal Care and Use Committee of the Faculty of Applied Health Sciences Technology at O6U developed the criteria for this experiment.
The animals in all groups fasted for 18 hours on day 13, the day before the last treatment. Those categorized into groups II, III, IV, and V were administered with paracetamol (1 g/kg body weight) 1 hour after the last dose of morin therapy on day 1432.
Blood sample treatment
Blood was drawn from the retroorbital vein of each animal and then placed in heparin-containing tubes. The heparinized blood was centrifuged at 1000 × g for 20 minutes, and the cholesterol33, triglyceride34, and HDL-C35 levels were measured in the separated plasma.
Liver sample preparation
The rats were sacrificed, and their livers were collected to assess the biochemical parameters. The liver homogenate was prepared using 25% W/V saline and glass homogenizer. Four aliquots of the homogenate were made.
The first aliquot was used to calorimetrically estimate the ALT36, AST36, ALP37, LDH38, tumor necrosis factor-alpha (TNF-α)39, and protein thiol40levels. The GSH level was calorimetrically measured in the second aliquot41 at 412 nm. The third aliquot was used to calculate the liver MDA level42 at 535 nm against a blank containing all reagents, except for the tissue homogenate. The fourth aliquot was minced with physiological saline and rinsed in ice-cold water. The supernatant was used to evaluate the SOD43 and CAT44 levels.
Primers used in real-time PCR
|
Genes |
Primer sequences |
|---|---|
|
NF-κB |
F: 5′-CATGAAGAGAAGACACTGACCATGGAAA-3’ |
|
R: 5′-TGGATAGAGGCTAAGTGTGACACG-3’ | |
|
NOX-2 |
F: 5′-TGAACAACAGCACTCACCAATGCC-3′ |
|
R: 5′-AGTTGTTGAACCAGGCAAAGGCAC-3′ | |
|
IL-6 |
F: 5'-GCCCTTCAGGAACAGCTATGA-3' |
|
R:5'-TGTCAACAACATCAGTCCCAAGA-3' | |
|
HO-1 |
F:5′-TGCTAGCCTGGTGCAAGATA-3′ |
|
R:5′-GCCAACAGGAAGCTGAGAGT-3′ | |
|
β-Actin (internal control for qRT-PCR) |
F: 5′-GGCTGTATTCCCCTCCATCG-3’ |
|
R:5′-CCAGTTGGTAACAATGCCATGT-3’ |
Real-time PCR
The total liver RNA was obtained using the TRIzol technique in accordance with the manufacturer’s instructions (Life Technologies Corp., Grand Island, NY). Thereafter, 1 µg RNA was combined with 0.5 mmol/L dNTP, 10 nmol/L dithiothreitol, 25 pg primer oligo (dT), and 200 units reverse RNase H superscript II in reaction buffer. The reactions were incubated for one cycle at 42°C for 2 minutes and then for another 50 minutes at 42°C, after which they were heated for 15 minutes at 70°C and chilled at 4°C.
Histological assessment
The liver tissues were divided into very thin slices (4 μm) and then fixed in 10% formaldehyde solution46. A light microscope was used to evaluate the histological alterations on H&E-stained slides.
Statistical analysis
The results were reported as means ± SDs. All data were statistically analyzed using SPSS version 1847 and one-way analysis of variance, with the statistical significance level set at P < 0.001.
Effect of morin and silymarin on plasma cholesterol, triglycerides and cholesterol-high density lipoprotein (HDL-C) in rats
|
No. |
Groups |
Cholesterol (mg/dl) |
Triglycerides (mg/dl) |
HDL-C (mg/dl) |
|---|---|---|---|---|
|
(I) |
Normal 1 % tween 80 |
153.2 ± 13.50 |
101.3 ± 6.85 |
34.12 ± 3.90 |
|
(II) |
Control Paracetamol (1 g/kg.b.w) |
261.7 ± 14.50a |
144.2 ± 9.61a |
23.29± 4.97a |
|
(III) |
Paracetamol + Morin (50 mg/kg.b.w.) |
184.2 ± 8.63ab |
121.2 ± 7.47ab |
29.48 ± 3.99ab |
|
(IV) |
Paracetamol + Morin (100 mg/kg.b.w.) |
165.8 ± 14.95b |
110.9 ± 11.55b |
31.86± 3.67b |
|
(V) |
Paracetamol + Silymarin (100 g/kg b.w.) |
157.6 ± 17.65bc |
108.5 ± 8.27b |
33.03± 3.43b |
Effect of morin and silymarin on liver alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH) in rats
|
No. |
Groups |
ALT (U/g tissue) |
AST (U/g tissue) |
ALP (U/g tissue) |
LDH (U/g tissue) |
|---|---|---|---|---|---|
|
(I) |
Normal 1 % tween 80 |
63.26 ± 6.24 |
129.0 ± 6.15 |
206.08 ± 12.77 |
312.1 ± 19.25 |
|
(II) |
Control Paracetamol (1 g/kg.b.w) |
103.1 ± 9.29a |
183.4 ± 8.40a |
276.89 ± 10.45a |
422.3 ± 14.19a |
|
(III) |
Paracetamol + Morin (50 mg/kg.b.w.) |
82.26 ± 7.13b |
137.19 ± 9.01b |
225.02 ± 8.06ab |
340.6 ± 15.22b |
|
(IV) |
Paracetamol + Morin (100 mg/kg.b.w.) |
68.58 ± 4.33bc |
134.5 ± 5.18b |
211.99 ± 12.53b |
311.9 ± 20.65b |
|
(V) |
Paracetamol + Silymarin (100 g/kg b.w.) |
64.62 ± 5.86bc |
129.8 ± 11.41b |
208.53 ± 8.92b |
308.8 ± 16.89bc |
Effect of morin and silymarin on hepatic protein thiols, reduced glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT) in rats
|
No. |
Groups |
Protein thiols (nmol sulfhydryl/mg protein) |
GSH (nmol/gm tissue) |
SOD (U/gm tissue) |
CAT (U/g tissue) |
|---|---|---|---|---|---|
|
(I) |
Normal 1 % tween 80 |
78.96 ± 5.57 |
26.41 ± 3.09 |
30.53 ± 5.65 |
99.81 ± 8.27 |
|
(II) |
Control Paracetamol (1 g/kg.b.w) |
52.65 ± 7.57a |
11.75 ± 1.68a |
10.86 ± 2.49a |
35.92 ± 4.46a |
|
(III) |
Paracetamol + Morin (50 mg/kg.b.w.) |
64.75 ± 5.47ab |
20.88 ± 2.63ab |
24.29 ± 3.42ab |
79.95 ± 7.23ab |
|
(IV) |
Paracetamol + Morin (100 mg/kg.b.w.) |
73.44 ± 6.99b |
22.37 ±3.13b |
27.36 ± 3.27b |
88.98 ± 8.44b |
|
(V) |
Paracetamol + Silymarin (100 g/kg b.w.) |
79.36 ± 7.41bc |
24.11 ±2.99 b |
29.13 ±2.63b |
92.26 ±11.50b |
Effect of morin and silymarin on liver malondialdehyde (MDA) and plasma tumor necrosis factor- alpha (TNF-α) in rats
|
No. |
Groups |
Hepatic MDA (nmole/g tissue) |
Plasma TNF-α (Pg/mL) |
|---|---|---|---|
|
(I) |
Normal 1 % tween 80 |
73.82 ± 6.93 |
36.64 ± 4.97 |
|
(II) |
Control Paracetamol (1 g/kg.b.w) |
154.0 ± 11.84a |
122.8 ± 10.28a |
|
(III) |
Paracetamol + Morin (50 mg/kg.b.w.) |
81.56 ± 7.92b |
50.20± 6.0ab |
|
(IV) |
Paracetamol + Morin (100 mg/kg.b.w.) |
76.07 ± 7.68b |
43.25± 4.38b |
|
(V) |
Paracetamol + Silymarin (100 g/kg b.w.) |
72.53 ± 5.10b |
40.91± 5.61b |

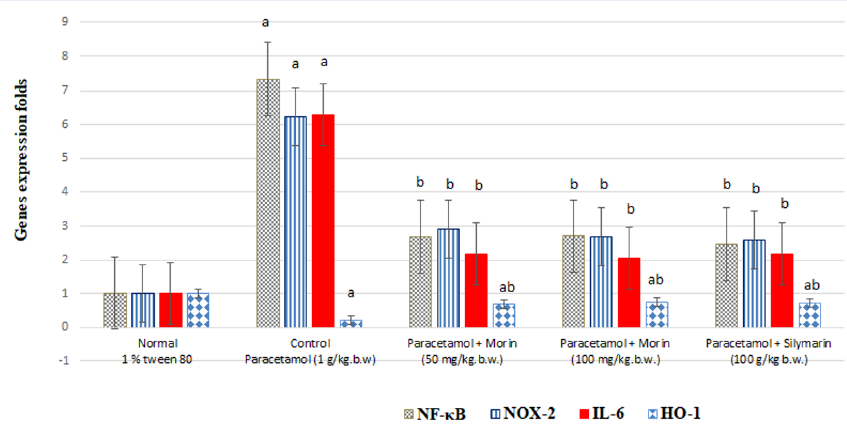
Effect of morin and silymarin on levels of liver nuclear factor kappa B (NF-κB), NADPH oxidase-2 (NOX-2), interleukin-6 (IL-6) and heme oxygenase-1 (HO-1) gene expression in paracetamol-treated rats. Representative bar diagram of three independent experiments is presented. a: significant with Group I; b: significant with Group II.

An agarose gel electrophoresis shows PCR products of liver nuclear factor kappa B (NF-κB), NADPH oxidase-2 (NOX-2), interleukin-6(IL-6), heme oxygenase-1 (HO-1) and beta actin (C) in different studied groups. M: DNA marker with 100bp.
Effect of morin and silymarin on hepatic histopathological changes in rats
|
No. |
Groups |
Congestion |
Lymphatic infiltration |
Necrosis |
Degenerated hepatocytes |
Pyknosis, karyolysis |
Fatty degeneration (steatosis) |
|---|---|---|---|---|---|---|---|
|
(I) |
Normal (1 % tween 80) |
- |
- |
- |
- |
- |
- |
|
(II) |
Control Paracetamol (1 g/kg.b.w) |
++ |
++ |
++ |
+++ |
+ |
+++ |
|
(III) |
Paracetamol + Morin (50 mg/kg.b.w.) |
+ |
+ |
+ |
++ |
+ |
++ |
|
(IV) |
Paracetamol + Morin (100 mg/kg.b.w.) |
+ |
+ |
- |
+ |
- |
- |
|
(V) |
Paracetamol + Silymarin (100 g/kg b.w.) |
+ |
+ |
- |
+ |
+ |
- |

Sections stained with hematoxylin and eosin (H&E; 200 X) histological examination of rat’s liver tissues of different groups compared to control group; (a), Group I: Normal control; (b), Group II: Was administrate paracetamol (1 g/kg.b.w.). (c), Group III: morin (50 mg/kg.b.w.) + Paracetamol (1 g/kg.b.w); (d), Group IV: Was administrate morin (100 mg/kg.b.w.)+ Paracetamol (1 g/kg.b.w); (e), Group V: Was administrate silymarin (100 mg/kg) + Paracetamol (1 g/kg.b.w). Abbreviations: PV: portal vein; BD: bile duct; CV: central vein; H: hepatocyte; A: artery; dh: degenerated hepatocyte; N: necrosis; Curved arrow: fatty degeneration; Arrows: kupffer cell; Star: lymphatic infiltration; Zigzag arrow: karyolysis; Arrow head: pyknotic nuclei; Curved arrow: binucleated cell.
Results
The plasma TC, TG, and HDL-C levels in the different groups of treated rats are shown in
Silymarin treatment (100 mg/kg body weight) reduced the liver AST, ALT, and ALP levels by 37.32%, 29.23%, 24.68%, and 26.87%, respectively, compared with paracetamol treatment.
The liver MDA and TNF-α levels significantly decreased by 50.60% and 64.78%, respectively, in the morin-treated rats compared with those in the paracetamol-treated rats (P < 0.001).
However, silymarin administration significantly decreased the liver MDA and TNF-α levels by 52.90% and 66.69%, respectively, compared with paracetamol administration alone (P < 0.001).
Figure 3 shows significantly increased (P < 0.001) liver NF-κB, NOX-2, and IL-6 levels (by 618.6%, 516.83%, and 523.76%, respectively) as well as a significantly decreased (P < 0.001) liver HO-1 level (by 77.23%) in the paracetamol (1 g)-treated rats compared with those in the control rats (P < 0.001), suggesting the presence of acute liver inflammation. Morin administration at 50 mg/kg body weight significantly decreased the expression of liver NF-κB, NOX-2, and IL-6 by 63.44%, 53.29%, and 65.5%, respectively, and significantly increased (P < 0.001) that of liver HO-1 by 204.34% compared with paracetamol administration (P < 0.001).
Morin administration at 100 mg significantly decreased the expression of liver NF-κB, NOX-2, and IL-6 by 63.16%, 57.14%, and 67.31%, respectively, and significantly increased (P < 0.001) that of liver HO-1 by 226.09% compared with paracetamol administration (P < 0.001).
Further, the silymarin (100 mg)-treated rats showed significantly decreased liver NF-κB, NOX-2, and IL-6 levels (by 66.30%, 58.42%, and 65.39%, respectively) and significantly increased (P < 0.001) liver HO-1 levels (by 208.70%) compared with the paracetamol-treated rats (P < 0.001).
The results of the agarose gel electrophoresis of NF-κB, NOX-2, IL-6, HO-1, and β-action via real-time PCR are presented in Figure 3.
In the histopathological evaluation of the liver sections, the control rats had a typical central vein and hepatocyte arrangement as well as a normal structure with no histological abnormalities. The nuclei of the hepatocytes were visible as dark red structures within the cells, whereas the cytoplasm was stained red. The sinusoids and typical portal area as well as the hepatic strands were observed from the margin of the lobule to the central vein. The number of binucleated and Kupffer cells was evaluated (
The liver sections of the paracetamol-treated rats showed an altered lobular shape and a nuclear disintegration in certain locations as well as disarrangement of normal hepatic cells, necrosis, and fatty degeneration. Enlargement and congestion of the hepatic central vein were observed, and lymphocyte infiltration was found in the portal area (
Histological examination of the hepatocytes of the groups showed signs of progress. The active euchromatic nuclei in the hepatocytes appeared to be in outstanding condition, while the hepatocytes and portal components appeared to be in good condition. Mild infiltrations of inflammatory cells were identified adjacent to the portal triads in the hepatic sections. The observed congestion was mild, and no evidence of fatty degeneration was found. The paracetamol- and morin-treated rats and silymarin-treated rats showed no necrotic changes compared with the paracetamol-treated rats (
Discussion
Paracetamol promotes hepatotoxicity by activating metabolic pathways. It is bio-transformed into NAPQI by the cytochrome P2E1 system in the endoplasmic reticulum. When NAPQI is combined with cellular lipids and proteins, mitochondrial dysfunction, lipid peroxidation, and oxidative stress occur48. The loss of Ca homeostasis eventually leads to cell death. The potential of a drug to suppress the aromatase activity of cytochrome P450, leading to liver regeneration, is known as a hepatoprotective action49, 50. In our study, oral paracetamol administration significantly increased the TC and TG levels and decreased the plasma HDL-C level, indicating the presence of liver toxicity. Morin and silymarin treatment reduced the plasma TC and TG levels while considerably increasing the HDL-C level. Generally, lipid deposition in the liver can occur because of an excessive supply of lipids or lipid deposition interference. Lipid-lowering drugs have been reported to have antioxidant properties that prevent lipid peroxidation, TC and TG elevation, and HDL-C induction by paracetamol51. Omidi . suggested polyphenols as beneficial agents in improving the lipid profile levels52.
In the present study, the liver ALT, AST, ALP, and LDH levels dramatically increased in the paracetamol-treated rats. This elevation may be attributed to the disruption of the liver cells as a result of necrosis53.
The liver cell damage induced by paracetamol overdose leads to NAPQI overproduction, hepatocyte necrosis, and liver enzyme level elevation54.
Our results indicate that morin and silymarin significantly reverse the enhancement of liver enzyme activity. The hepatoprotective activity of morin has been identified in certain toxicant and models of induced liver fibrosis55, 56, 57, while that of polyphenols is common in experimental and clinical studies. Morin and silymarin are used as a hepatoprotective and antioxidant drug58.
Our study findings agree with those of Asmah59 that polyphenols have a hepatoprotective impact and considerably reduce the liver enzyme levels in animal models.
The current work investigated paracetamol-induced liver necrosis . Endogenous reactive oxygen species are produced by NAPQI linked to the thiol group of GSH, resulting in lipid peroxidation and cell death60. Our analyses revealed that the paracetamol-treated rats had lower protein thiol, GSH, SOD, and CAT levels.
Oral morin treatment significantly improved the liver protein thiol, GSH, SOD, and CAT levels. Morin has a wide range of antioxidant activities because it has a hydroxyl group (-OH) and a double bond between the C2 and C3 atoms that activates the double bond61. Its anti-lipid peroxidation potential is caused by the presence of 2 -OH at the 2′ and 4′ locations of the B ring62, 63, 64. Furthermore, the antiradical action of related polyphenols is thought to be predominantly due to -OH61.
The free radical scavenging activity of morin against ABTS and cytoprotective activity against ABTSradicals in tissue fibroblasts were evaluated and found to increase the superoxide dismutase activity and reduce intracellular ROS generation.
Herein, paracetamol-induced lipid peroxidation was detected in the rats, as demonstrated by the increased MDA levels and decreased GSH, SOD, and CAT levels in the liver tissues, all of which are key antioxidant indicators. MDA immunohistochemistry was found to be associated with hepatocyte necrosis in the central zone at early time points. Attenuation of inflammatory markers is mediated by a similar mechanism65. We found an elevation of the liver TNF-α levels that indicated the presence of oxidative stress and inflammation in the liver of the paracetamol-treated rats. However, oral morin and silymarin administration to the paracetamol-treated rats significantly decreased the MDA and TNF-α levels compared with paracetamol administration only. The current results on morin and silymarin treatment could be attributed to their antioxidant and membrane-stabilizing capabilities as well as a probable influence on paracetamol metabolism, as suggested by our findings on phase I bio-transformation enzymes involved in paracetamol bioactivation60, 61.
Morin enhanced the hemodynamic profile and inhibited the inflammatory marker pathways in a dose-dependent manner66.
We observed that the paracetamol-treated rats showed increased NF-κB, NOX-2, and IL-6 levels and decreased HO-1 levels. However, morin and silymarin administration depleted the expression of NF-κB, NOX-2, and IL-6 and alleviated the significant increase in the OH-1 level compared with paracetamol administration only.
Morin downregulated HO-1, which is linked to decreased Nrf2 expression and phosphorylation and improved Keap1 expression67. Morin administration in chemotherapy also had cytoprotective effects68.
The effect of morin in suppressing LXR signaling may help reduce liver steatosis and other metabolic disorders69. Morin can also improve the degree of gene expression of inflammatory mediators and controlled liver function induced by anticancer medication70. All biochemical parameters, especially NF-κB, NOX-2, IL-6, and HO-1, proved the antioxidant and anti-inflammatory mechanism of morin in the amelioration of paracetamol-induced toxicity.
To our knowledge, our study may be the first to evaluate the regulatory activity of morin for NF-κB, NOX-2, IL-6, and HO-1 in a model of paracetamol-induced liver fibrosis.
Conclusions
Morin protects against paracetamol-induced liver fibrosis by ameliorating the antioxidant and inflammatory biomarker levels. Its scavenging activity improves the expression of NF-κB, NOX-2, IL-6, and HO-1 in the liver.
Acknowledgments
None.
Author’s contributions
All authors equally contributed to this work. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

