Roles of hypoxia in tumor progression and novel strategies for cancer treatment
- Laboratory of Stem Cell Research and Application, University of Science, Ho Chi Minh City, Viet Nam
- Vietnam National University Ho Chi Minh City, Ho Chi Minh City, Viet Nam
Abstract
The metabolic process of normal cells in general and of cancer cells in particular requires an important molecule—oxygen. In tumors, the oxygen level tends to decrease gradually from the outer layers to the central core, leading to a condition termed ``hypoxia.'' Changes in the oxygen level modify the signaling pathways and metabolic activities of cancer cells. Basically, tumor development is divided into three stages: initiation, promotion, and progression. Among them, the effects of hypoxia are most evident during tumor progression. In this review, we summarize previous findings on the mechanisms underlying hypoxia-induced alterations in the expression of genes and proteins associated with hypoxia-inducible factors (HIFs), which play a central role in the development of malignancy in many types of cancer. We also present the latest evidence on HIF-targeted cancer treatment that yields positive outcomes in vitro and in vivo.
Introduction
Cancer is a group of diseases with a leading mortality rate and a high recurrence rate owing to the absence of effective targeted therapies. Therefore, in-depth understanding of its characteristics is crucial in treating this fatal disease.
Recently, hypoxia has emerged as a novel candidate in targeted therapy for cancer. Hypoxia exists in solid tumors (e.g., breast, pancreatic, liver, lung, cervical, ovarian, or colon cancer)1 as well as liquid tumors (e.g., leukemia)2. It has been shown to influence the malignant properties of tumors and to induce resistance to cancer treatment3. Signaling pathways related to hypoxia-inducible factors (HIFs) play a central role in metabolism adaption of cancer cells4, contribute to adaptive immune escape5, regulate angiogenesis, and promote cell proliferation and survival and tumor progression6. Hence, hypoxia is considered to affect several hallmarks of cancer7.
Maintaining the level of cellular oxygen is crucial to cell fat, since oxygen molecules act as terminal acceptors in the electron transmission chain to create ATPs during oxidative phosphorylation8. This process leads to reactive oxygen species production, consequently causing cell death owing to DNA damage and genomic instability8, 9.

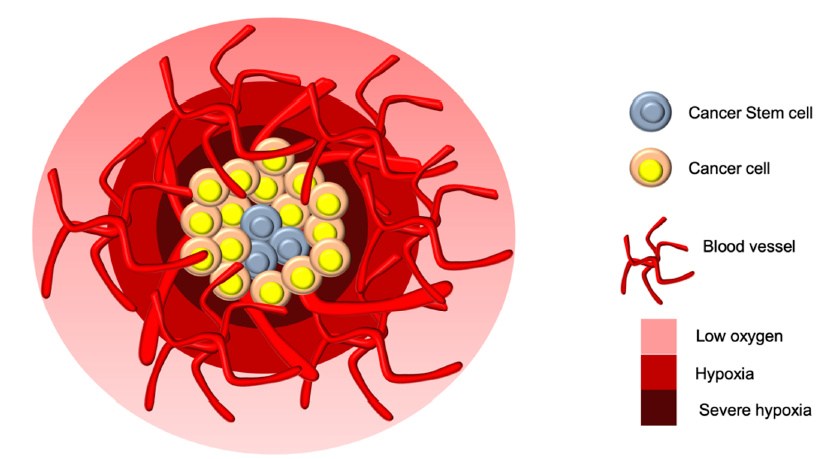
Oxygen concentration-based partional structure in cancer tumors with possible niche for cancer stem cells in the center necrotic core.
The tumor oxygen level typically ranges from 0.3% to 2.2% in various types of cancer10, and the oxygen level gradient reduces gradually from the periphery to the center of tumors (Figure 1)11. If an increased oxygen level is required in tumors, they may induce angiogenesis with dysfunctional blood vessels, consequently leading to different hypoxic statuses and tumor aggressiveness8. Rates of cell proliferation higher than those of new vessel formation in tumors lead to unequal perfusion and acute hypoxia among tumor regions. Gradually, strongly proliferative cancer cells accumulate and settle in areas unreachable by nutritive blood vessels, thereby causing chronic hypoxia. Both acute and chronic hypoxia can contribute to therapy resistance12.
In the present review, we summarize several major roles of hypoxia in tumor development and provide the latest information on hypoxia-targeted cancer treatment.
Hypoxia and tumor initiation and promotion
Tumors are formed owing to an uncontrolled division of cells. This loss of control is caused by errors in genomes and chromosomes through chemical, physical, or biological agents. Defects largely due to DNA replication and repair lapses, oncogene mutations, chromosomal instabilities, and tumor suppressor gene mutations are fundamental for tumor initiation13, 14.
Several research has shown a relationship between chronic hypoxia and tumor initiation both and HIF-1α reduces the ability to repair DNA damage and causes gene mutations in cells grown under hypoxic conditions. Under severe hypoxic conditions, replication is unstable, which may lead to some errors in DNA replication15. Previous studies have revealed genomic instability of prostate cancer cells cultured under hypoxic conditions, which substantially contributes to tumor initiation. HIF-1α also upregulates human telomerase reverse transcriptase to restore the telomere, leading to intractable cell proliferation and triggering tumor promotion and formation16. It is involved in the regulation of cell death through various mechanisms in many cell types17. Depending on the conditions of action, apoptosis is either activated or inactivated with the regulation of the expression of p53 or BCL-2 family signaling pathways17, 18. Under chronic hypoxic conditions, HIF-1α upregulates p53 via various mechanisms but mostly inhibits apoptosis through indirect effects on MDM2 to inactivate this gene18. HIF-1α also inhibits BAX and activates BCL-2 to prevent mitochondrial -cytochrome release and inactivate apoptosis17. Based on these correlational factors between hypoxic conditions and basic factors of tumor formation, hypoxia may induce tumor initiation.
Hypoxia and tumor progression
Hypoxia and angiogenesis
To increase the intra-tumor oxygen level, primary tumors have to induce angiogenesis and form new vessels supplying oxygen-rich blood from the arteries for feeding. Angiogenesis is of great importance in tumor growth. In the absence of angiogenesis, defined as the process that creates new arteries and veins from preexisting blood, tumors remain dormant, maintaining an equal state between cell survival and death19. Genes associated with angiogenesis, such as vascular endothelial growth factor (VEGF), angiopoietin (ANG), and platelet-derived growth factor (PDGF), are some representative proteins in the downstream targets of HIFs20.
VEGF has been isolated, identified, and studied and in mammals in the early 1990s. German scientists have claimed that VEGF mRNA is overexpressed during the formation of new blood vessels in mouse embryos, which indicates that VEGF and its receptors VEGF-R1 and VEGF-R2 are major factors in mammalian angiogenesis21, 22. Previous studies suggest a link between HIF-1α and VEGF not only in cancer23 but also in other diseases24. Under the phosphorylation of specific protein 1 (Sp1) and HIF subunit and recruitment of VEGF promoters, transcription is triggered under the effect of the Ras/MEK/extracellular signal-regulated kinase (ERK) pathway. The expression of VEGF-A mRNA can be increased by the PI3K/AKT pathway and modified by activator protein-125. The phosphorylation of HIF-1α and coactivator p300 induces transcriptional activation via ERK, whose pathway attracts the connection between RNA polymerase II and VEGF promoters. At the other regulation levels, the transcription of VEGF mRNA is stabilized through stress-activated kinase p3826. At the translational level, the internal ribosome entry site (IRES) sequences are inserted in the middle of the 5’ non-coding regions of VEGF-A mRNA27 and HIF-1α mRNA28. The interference of the IRES between these two components accounts for the translation under hypoxic conditions and nutrient deprivation. Normally, the cap-dependent translation often halts in such hypoxic and nutrient-deprived conditions. However, VEGF is considered an “existing” factor that continues to be translated under hypoxia29. Some studies have demonstrated the complementary roles of HIF-1α and HIF-2α in angiogenesis. In particular, HIF-1α enhances vessel growth, while HIF-2α promotes vessel maturation30, 31. Recently, several new transcription factors, microRNAs (miRNAs), and RNA-binding proteins have been demonstrated to be crucial for axis HIF-1α/VEGF signaling in tumor progression. Zinc finger homeobox32, Bcl-2-associated transcription factor 133, and miRNA-574-5b34 are typical examples of factors that regulate the ability to induce angiogenesis under hypoxic conditions within tumors.
In addition to VEGF, the ANG protein family (ANG-1, ANG-2, and ANG-4) also contributes to many aspects of angiogenesis35, 36. Early upregulation of ANG proteins has been observed in hepatocellular carcinoma37, glioblastoma38, 39, and squamous cell carcinoma40, in which their effect on tumor angiogenesis differs from that of VEGF during tumor progression. ANG-1 or ANG-4 and ANG-2 affect Tie-2 receptor, a tyrosine kinase receptor selectively expressed in the vascular endothelium41, on opposite directions. While ANG-1 or ANG-4 binds to and phosphorylates Tie-2, resulting in vessel stabilization and remodeling, ANG-2 acts as an antagonist of Tie-2 to destabilize and regress the vessels36. However, this inhibitory effect of ANG-2 can be prevented under the presence of VEGF derived from avascular and hypoxic tumors owing to tumor vessel decline41. The ANG-1/ANG-2 ratio markedly contributes to physiological angiogenesis42, since a reduction in this ratio under hypoxia with an increased level of VEGF results in tumor vessel formation39, 43. ANG-2 secreted from human melanoma cells is also believed to be a prognostic marker for metastasis44, indicating that it plays crucial roles not only in angiogenesis but also in other aspects during tumor progression. Thus, blocking the expressions of VEGF and ANG-2 is a new and effective strategy to lessen brain and lung metastases45, 46.
PDGF, which has been first identified in platelets, is an important factor in blood vessel regulation47. PDGF isoforms interact with their tyrosine kinase receptors to perform various physiological functions48. Gene mutations of PDGF receptors have been observed in different malignant tumors48, which may lead to disturbances in related signaling pathways49. In osteosarcoma, an increased level of PDGF-BB and its receptor is transcriptionally dependent on HIF-1α to promote cell proliferation and migration under hypoxic conditions50. Overexpression of HIF-1α is related to an increased expression of PDGF-BB in invasive breast cancer cells51, which contributes to lymphatic vessel density and lymph node metastasis52. The proportional relationship between HIF-1α and PDGF-BB is regulated by AKT, forming an autocrine loop that may increase cisplatin resistance in hepatocellular carcinoma53. Similarly, hypoxia was found to enhance the HIF-1α/PDGF-D/PDGFRα/AKT pathway, which accelerated cell growth and invasion in glioblastoma54. Only HIF-1α appears to play a central role in the regulation of PDGFs, since no studies have confirmed a link between other HIFs and PDGFs.
Hypoxia and epithelial–mesenchymal transition
Epithelial–mesenchymal transition (EMT) is crucial in the invasion and metastasis of tumors, as cells lose their epithelial differentiation to acquire the mesenchymal phenotype, which allows them to detach from primary tumors and disseminate into stromas55. Hypoxia is notably a hallmark of cancer and is believed to induce EMT via HIFs in various types of cancer20, 56, 57, 58, 59, 60. HIF-1α or HIF-2α upregulates the Wnt/-catenin signaling pathway to enhance the biological features related to EMT, which helps tumor cells survive and proliferate during hypoxia56, 58. In contrast, HIF-3α isoform 2 binds to and destabilizes -catenin to inhibit Wnt signaling, which is involved in stem cell renewal and tissue homeostasis61.
Loss of E-cadherin and switching to N-cadherin are also considered a marker of EMT owing to their main role in cell–cell adhesion in epithelial cell membranes62. Twist63, zinc finger E-box binding homebox (ZEB) 1/264, 65, Snail66, and Slug67, 68 are major transcription factors directly or indirectly responsible for this cadherin switch in metastatic cancer. Consequently, blocking these factors has been recently suggested as a new strategy for preventing metastatic cancer69, 70, 71, 72. Their expressions seem to be affected by HIF-1 signaling. In hypoxic HeLa cervical cancer cells, the expression levels of miR-21 and miR-210 are increased, thus targeting and downregulating HECT domain E3 ubiquitin ligase 1 and consequently increasing Snail expression to attenuate the E-cadherin level73. MiR-21, miR-210, and several other miRNAs act as downstream molecules of HIF-1α in relation to cancer cell viability and migration74, 75, 76. Slug is also an E-cadherin direct regulator, which is upregulated by dimethylation of lysine demethylase 3A or binding of OCT4B under hypoxic conditions77, 78. Meanwhile, hypoxia enhances Bcl-2–Twist interaction by facilitating the binding of Bcl-2 to Twist, forming a protein complex and then targeting Bmi-1 to cause changes in EMT-related proteins in cancer cells79, 80. Interestingly, HIF-2α stimulates Twist-2 to bind to the E-cadherin promoter through the P2 region and activates EMT in pancreatic cancer81. Additionally, HIF-1α directly inhibits E-cadherin via miR-421 signaling or miR-205/ASPP2 axis in cancer cells82, 83. It also controls EMT and the stem cell-like phenotype of liver cancer cells via miR-19184.
Transforming growth factor (TGF)-β, released by cancer cells, stromal fibroblasts, and other cells in the tumor microenvironment, has a key role in promoting EMT during cancer progression and metastasis85. The phosphorylation of the binding between TGF-β and its receptor TGFR1 and TGFR2 promotes the activation of Smad2 and Smad3, which form a trimer with Smad4. This complex then displaces into the nucleus, in which a synergic action of the DNA binding transcription factors SNAIL, ZEB, and TWIST connects to the regulation of the target genes of TGF-β86. The activity of EMT-related transcription factors can be increased during the combination of the Smad complex with any targeted effects87. The activation of co-bindings between Smad3 and Smad4 caused by TGF-β leads to several events, including the repression of E-cadherin and inhibition of the gene expression owing to the dual interaction with SNAIL188, increasing the expression of TWIST owing to the dual interaction between Smad3 and Smad4 originating from the interaction with activating transcription factor 3 to suppress the expression of ID189. TGF-β1 triggers the downregulation of prolyl hydroxylase-2 via a Smad-dependent signaling pathway, leading to the accumulation of HIF-1α and EMT90.
Notch is considered a major receptor involved in the induction of the EMT signaling pathway under hypoxia. During downstream regulation, Notch regulates SNAIL in two different synergistic methods: direct or indirect regulation. The recruitment of the Notch intracellular domain to the SNAIL promoter leads to direct upregulation of SNAIL with the combination of the transcriptional complex with HIF-1α and to indirect upregulation of SNAIL with an increase in the levels of LOX protein stemming from the combination of HIF-1α with the LOX promoter 91. SNAIL and SLUG, the two transcriptional repressors involved in EMT, also aim at the Notch signaling pathway92. In breast cancer cells, an increase in the expression of SNAIL and SLUG, which follows the accumulation of HIF-1α and HIF-2α, reduces the expression of E-cadherin93. Notch activity has also been reported in lung cancer stem cells (CSCs) via the expression of spheroid growth in cell cultures, high rate of chemoresistance, and tumor formation in the cell injected to the CSCs of NOD/SCID mice94. In addition, the Notch signaling pathway can induce cell cycle arrest and apoptosis, which are key steps in carcinogenesis95, 96, 97.
Hypoxia and tumor invasion and metastasis
Malignant cancer is characterized by the formation of secondary tumors through invasion and metastasis. Under hypoxic conditions, HIF-1α and HIF-2α, controlled by the c-Jun NH2-terminal kinase pathway, are involved in the migration of gastric cancer cells98; thereby, HIFs play a crucial role in this oncological process.
Changes in the HIF-1α level strongly induce the expression of mRNAs encoding for urokinase-type plasminogen-activator receptor, metalloproteinase-2 (MMP-2), and cathepsin D, proteins involved in the degradation of the extra-cellular matrix, and disrupt basement membrane invasion in colon cancer99, esophageal carcinoma100, and prostate cancer101. Via ZEB-2, HIF-1α downregulates EphrinB2, whose low expressions cause tumor invasiveness in human glioma102. Recently, the expression of other downstream targets of HIF-1α, including right open reading frame kinase-3 involved in the organization of actin cytoskeleton103, v-maf musculoaponeurotic fibro-sarcoma oncogene homolog F104, and SP1105, has also been demonstrated to increase when cancer cells are exposed to low oxygen levels, which promotes cell invasion and metastasis. In breast cancer, hypoxia induces the upregulation of ADAM12, thereby activating EGFR/FAK signaling and inducing lung metastasis in SCID mice106, which may be associated to poor outcomes. In hypoxic non-small-cell lung cancer, Twist not only inhibits E-cadherin expression but also directly increases the cancer cell motility rate107. Overexpression of Twist upregulates AKT2 in the late stages of breast cancer108, inducing its aggressiveness, and enhances Tcf-4/Lef DNA binding, promoting invasion and cell migration in gastric cancer109. Such overexpression has been proven to be directly induced by the upregulation of HIF-1α under hypoxia110.
HIF-2α also promotes invasion and metastasis of cancer cells via the upregulation of Twist and CXCR4 in papillary thyroid carcinoma111. In hepatocellular carcinoma, Wang found that HIF-2α directly regulates the transcription of stem cell factor, increasing the expression of MMP-2 and promoting cancer cell invasion112. The E2F3 transcriptional regulatory pathway is considered to induce an overexpression of HIF-2α owing to many steps in cancer progression113. Furthermore, HIF-2α is found to be highly expressed compared with HIF-1α when ovarian cancer cell lines enter the hypoxic state and become more invasive114. Down- or upregulation of both HIF-1α and HIF-2α is related to a higher or lower expression of insulin-like growth factor binding protein-3, respectively114, which has been demonstrated to suppress tumor angiogenesis and growth arrest115. HIFs can either affect or be affected by upstream and downstream factors to regulate the invasive and metastatic capabilities of cancer cells.
HIF-3α has recently been demonstrated to regulate pancreatic cancer invasion and metastasis via RhoC/ROCK1 signaling116. Further experiments are required to confirm the role of HIF-3α in cancer progression.
Hypoxia induces autophagy in cancer cells to adapt to cancer therapies
|
Type of cancer |
Related hypoxia genes |
Autophagy markers |
Therapy-resistance |
Referrence |
|---|---|---|---|---|
|
Bladder cancer |
HIF-1 |
LC3-II |
Gemcitabine |
|
|
Bladder cancer |
HIF-1 |
Beclin-1 |
Cisplastin |
|
|
Hepatocellular carcinoma |
N/A |
FOXO3a |
Sorafenib |
|
|
Hepatocellular carcinoma |
N/A |
LC3-II |
Cisplastin Epirubicin Gemcitabine Mitomycin |
|
|
Lung cancer |
N/A |
N/A |
Cisplastin |
|
|
Lung cancer |
N/A |
N/A |
Radiotherapy |
|
|
Lung cancer |
HIF-1 |
LC3-II Beclin-1 p62 |
Radiotherapy |
|
|
Colon cancer |
HIF-2 |
N/A |
5-flourouracil CCI-779 |
|
|
Colon cancer |
HIF-1 |
Beclin-1 Atg12 LC3-II |
Radiotherapy |
|
|
Ovarian cancer |
HIF-1 |
N/A |
Cisplastin |
|
|
Cervical cancer |
HIF-1 |
LC3-II Beclin-1, Atg12-Atg5 Atg7 p62 |
Paclitaxel |
|
|
Breast cancer |
N/A |
Beclin-1 Atg12-Atg5 Atg7 |
Ionizing radiation |
|
|
Osteosarcoma |
HIF-1 |
LC3 |
Radiotherapy |
|
|
Glioblastoma |
HIF-1 |
Atg5 |
Temozolomide |
|
|
Astrocytoma |
HIF-1 |
Atg5 |
Temozolomide |
|
Hypoxia and immune escape from cancer
The immune system regulates tumor biology by inhibiting the malignant characteristics of tumors. Immune escape from cancer suppresses the effects of immune cells and allows resistance to the cytotoxicity of immune effectors, and hypoxia may contribute to this process129. Under hypoxic conditions, tumor cells release immunosuppressive molecules129. In hepatocellular carcinoma, hypoxia positively influences the expression of S100, heat shock proteins, and high-mobility group B1 in tumor cell-released autophagosomes, which induces the production of IL-10 and B-cells with the ability to hamper T-cell proliferation and function130. As T-cells can kill tumor cells by binding to their receptors131, and they are considered main targets in cancer immunotherapy132, their inhibition may decrease the effectiveness of cancer treatment. TGF- , a multifunctional cytokine and T-cell proliferation suppressor133, is also highly expressed in gastric cancer134 and glioblastoma135, along with HIF-1α. TGF- converts naive T-cells to regulatory T-cells136, thereby ameliorating the function of natural killer cells137, and remarkably suppresses the maturation of dendritic cells138, which may reduce the effectiveness of the antitumor activities of the immune system. Nevertheless, hypoxia sometimes supports T-cell development via stimulation of CD137 on the T-cell surface in patients with colon carcinoma139 or in anti-VEGF-treated mice140.
Hypoxia also alters cell surface molecules that may bind to immune checkpoints in T-cells to induce immune escape. Programmed cell death protein-1 and cytotoxic T lymphocyte-associated antigen-4 are two main immune checkpoints in T-cells that downregulate T-cell pathways when conjugated to their ligands (PD-L1 and PD-L2 or CD80 and CD86, respectively)141. Multiple drugs with the ability to inhibit these two molecules have been studied and developed to treat many types of cancer; these drugs include ipilimumab142, nivolumab143, pembrolizumab144, atezolizumab64, 145, 65, 146, and tremelimumab147. Both HIF-1 and HIF-2α directly bind to hypoxia response element-4 in the PD-L1 proximal promoter, thereby upregulating the expression of this ligand in tumor cells and mediating the suppression of T-cells148, 149. Enhancer of zeste homolog-2 potentially affects the expression of PD-L1 via HIF-1α150. HIF-1α-induced PD-L1 increases the resistance to cytotoxic T lymphocyte-mediated lysis and leads to T-cell apoptosis in cancer cells5.
Hypoxia and therapy resistance
HIFs have been confirmed to be related to miRNAs in promoting cancer therapy. Many miRNAs control the transcriptional activity of HIFs. For instance, miR-199a is suggested to be a tumor suppressor, since its downregulation under hypoxic conditions results in an overexpression of HIF-1α and is associated with increased cisplatin resistance in osteosarcoma cell lines151. While hepatocellular cancer cells are resistant to 5-flourouracil, overexpression of miR-183 significantly reduces the expression of isocitrate dehydrogenase 2 or suppressor of cytokine signaling 6, which is related to the upstream signaling of HIF-1α152. On the contrary, upregulation of miR-21 may lead to an overexpression of HIF-1α and promote resistance to cisplatin in lung adenocarcinoma cell line A54966. Further, miR-193a-3p negatively regulates the expression of SRSF2 via the hypoxia signaling pathway to promote radio-resistance among nasopharyngeal cancer cell lines153. In general, miRNAs either directly or indirectly increase the expression of HIFs, thereby causing therapy resistance in different cancer cell lines.
Conversely, some miRNAs have been reported to act as downstream factors of HIFs. In bladder cancer, cisplatin attacks and upregulates HIF-1α, increasing the level of miR-424. MiR-424 may mediate the suppression of UNC5B and SIRT, decreasing the number of apoptotic cancer cells induced by cisplatin154. In contrast, HIF-1α upregulated in hypoxic colorectal cancer cells directly inhibits miR-338-5p and activates the IL-6/STAT3/Bcl-2 pathway, consequently causing resistance to cisplatin155. Hypoxia also diminishes the efficacy of sorafenib in treating hepatocellular carcinoma by inhibiting the androgen receptor and miR-520f-3p to increase the expression of SOX9 and modulate the stem-like phenotype within liver cancer cells156. However, the link between hypoxia and miRNAs requires further clarification. Nonetheless, both are known to be involved in cancer therapy resistance.
Autophagy is another mechanism of hypoxia that mediates therapy resistance in cancer cells. Several studies have reported the effect of hypoxic conditions on the ability of cancer cells to resist therapy (). HIFs, mainly HIF-1α, either activate or inactivate transcription factors participating in autophagy. In glioblastoma and astrocytoma cells, HIF-1α negatively regulates miR-224-3p, thereby enhancing the expression of Atg5 and FIP200, inducing autophagy, and postponing chemosensitivity and 128, 157. Similar results were obtained in colorectal cancer cells and tissues. HIF-1α downregulates the expression of miR-20a to mediate hypoxia-induced autophagy via the upregulation of Atg5/FIP200 signaling158. It also positively controls astrocyte elevated gene-1 in T-cell non-Hodgkin’s lymphoma and promotes autophagy-induced chemoresistance under hypoxia159. Since chemotherapy and radiotherapy usually induce apoptosis160, 161, tumor cells activate autophagy to digest damaged cellular components. Hypoxia-induced autophagy either positively or negatively contributes to the regulation of immune escape from cancer129.

New and novel strategies in drug development targeting HIFs in cancer treatment during last decade.
Targeting HIFs for cancer treatment
Owing to the crucial roles of HIFs, especially HIF-1α, in tumor development, cancer treatment has recently involved the use of HIF inhibitors. There are two types of HIF inhibitors: direct and indirect162. However, regardless of the type, the ultimate purpose of these inhibitors is to completely block HIF-related signaling pathways, thereby reducing the malignant properties of cancer tumors.
Temsirolimus and everolimus are examples of indirect HIF inhibitors clinically used in treating renal cell cancer67. These drugs inhibit the activity of mTORC1, thereby destabilizing HIFα signaling pathways67. Recently, natural compounds isolated from plants have been utilized in cancer drug development. For instance, cardamonin, a chalcone extracted from , inhibits HIF-1α activity and tumor angiogenesis in breast cancer xenograft models through the mTOR/P70S6K pathway163. Apigenin inhibits HIF-1α protein expression by downregulating the PI3K/AKT pathway and enhancing the stability of p53 in ovarian, prostate, and breast cancer cells164. Genipin, derived from , inhibits HIF-1α accumulation under hypoxic conditions in various cancer cell lines and prevents invasion of colon cancer cells by downregulating the ERK signaling pathway68. Even a short exposure to shikonin, extracted from , suppresses NF-kB activity and thereby downregulates HIF-1α protein during lymphangiogenesis165. These suggest the great potential of using plant-derived compounds in cancer treatment targeting HIF signaling pathways.
Generally, direct HIF inhibitors downregulate the expression of HIFs by suppressing the transcriptional and translational activities or disrupting the connection with other proteins67. Instead of reducing the HIF-1α level, arylsulfonamide KCN1 interferes with the interaction between HIFs and p300/CBP to inhibit glioma growth and 69. In a pilot trial, the use of EZN-2968 in 10 patients with different types of cancer yielded positive outcomes, since it decreased the HIF-1α mRNA and protein levels166. Hypoxia contributes to tumor malignancy by controlling downstream targets, with HIFs playing a central role. Aminoflavone (AF), which has been studied in phase II clinical trials on cancer treatment, also specifically inhibits HIF-1α transcriptional activity and avoids HIF-1α and HIF-2α in several cancer cell lines167. It diminishes tamoxifen resistance by suppressing α6-integrin and inducing BAX expression in tamoxifen-resistant breast cancer cells168. Belzutifan, an advanced pharmacologic agent blocking HIF-2α activity by allosterically interfering with its interaction with HIF-1169, is safer and more effective for patients with von Hippel–Lindau disease-associated renal cell carcinoma170; it was approved by the United States Federal Drug Administration for use in patients with non-metastatic tumors171. Sanguinarine blocks HIF-1α translocation and reduces hypoxia-induced EMT marker and VEGF levels in hepatocellular carcinoma172. Panaxadiol, isolated from , inhibits PD-L1 expression by blocking HIF-1α protein synthesis and restores T-cell activity to kill colon cancer cells in co-culture models173. These examples propose a novel strategy for drug combinations in futuristic cancer treatment (Figure 2).
Conclusion
Hypoxia plays a pivotal role in cancer progression. Regulation of HIFs under hypoxic conditions changes the expression of their target genes and related pathways to cause tumor aggressiveness, including angiogenesis, EMT, metastasis, immune escape, and therapy resistance. New methods developed to specifically inhibit HIFs and their signals have initially demonstrated their effects and . Further clinical trials are necessary to confirm the roles of these compounds in cancer treatment targeting hypoxia.
Abbreviations
AKT: Ak strain transforming, ANG: angiopoietin, Atg: autophagy-related gene, BAX: BCL-2-associated X, BCL-2: B-cell lymphoma 2, CD: cluster differentiation, CSC: cancer stem cell, EMT: Epithelial–mesenchymal transition, ERK: extracellular signal-regulated kinase, HIF: hypoxia-inducible factor, IL: interleukin, LC3: light chain 3, MDM2: Murine double minute 2, miR: microRNA, mTOR: mammalian target of rapamycin, N/A: not available, NOD/SCID: non-obese diabetic/Severe combined immunodeficiency, PD-L1/2: Programmed death-ligand ½, PDGF: platelet-derived growth factor, PI3K: phosphoinositide 3-kinase, TGF-beta: transforming growth factor beta, VEGF: vascular endothelial growth factor, ZEB: zinc-finger E-box binding-homebox
Acknowledgments
None.
Author’s contributions
Bui Dinh Khan took the main responsibility for layout and content of the manuscript. Tran Ngo The Nhan, Hoang Nguyen Minh Chau and Nguyen Thi Yen Nhi equally contributed to this work. All authors read and approve the final version of the manuscript.
Funding
This paper is funded by Vietnam National University Ho Chi Minh City (VNUHCM) under grant number C2020-18-27.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

