
Tualang honey-mediated silver nanoparticles attenuate hippocampal oxidative stress in kainic acid-induced male rats
- Department of Chemical Pathology, School of Medical Sciences, Universiti Sains Malaysia, Health Campus, 16150 Kubang Kerian, Kota Bharu, Kelantan
- Department of Basic Medical Sciences, Kulliyyah of Medicine, International Islamic University Malaysia, Bandar Indera Mahkota, 25200 Kuantan, Pahang, Malaysia
- Centre for International Collaboration and Research, Reva University, Rukmini Knowledge Park, Kattigenahalli, Yelahanka, Bangalore, Karnataka, 560064, India
- PET Image core, Houston Methodist Research Institute, Houston Texas, 77030, United States of America
- Department of Anatomy, School of Medical Sciences, Universiti Sains Malaysia, Health Campus, 16150 Kubang Kerian, Kota Bharu, Kelantan, Malaysia
Abstract
Introduction: Kainic acid (KA) has been widely used to study the mechanism of excitotoxicity-induced neurodegeneration and to investigate neurodegenerative therapeutic intervention. The present study aimed to investigate the protective effects of Tualang honey-mediated silver nanoparticles (THSN) against oxidative stress in the hippocampus of KA-induced rats.
Methods: Male Sprague Dawley rats (n = 72) were randomized into six groups: i) control, ii) THSN 10 mg, iii) THSN 50 mg, iv) KA only, v) THSN 10 mg + KA, and vi) THSN 50 mg + KA. The animals were administered distilled water or THSN (10 or 50 mg/kg), according to their respective groups, five times at 12 h intervals before being injected subcutaneously with saline or KA (15 mg/kg). Animals were sacrificed after 24 h and 5 days of KA induction. Malondialdehyde (MDA), total nitrate/nitrite (NOx), protein carbonyl (PCO), glutathione (GSH), total antioxidant status (TAS), and catalase (CAT) activity in the hippocampal tissue were measured using commercially available ELISA kits.
Results: THSN pre-treatments significantly improved oxidative status in the hippocampus by decreasing the MDA, NOx, and PCO levels while increasing the levels of GSH, TAS, and CAT activity.
Conclusion: THSN attenuated the KA-induced oxidative stress in the rat hippocampus through its antioxidant effects.
Introduction
Oxidative stress, a condition in which antioxidant defenses of the body are overcome by oxidants (free radicals), is known to damage biomolecules and several cellular components, which can potentially impact the whole organism1. It has been shown that oxidation induced by the presence of free radicals can damage plasma membranes, cellular proteins, lipids, and even DNA, which can initiate the development of many diseases if not properly controlled, including neurodegenerative diseases (., Parkinson’s disease, Alzheimer’s disease, ischemic stroke), cardiovascular disease, and cancer2. Fortunately, the human body is equipped with an antioxidant defense system to counteract the negative actions of oxidants, which can be categorized into enzymatic and non-enzymatic antioxidants. Enzymatic antioxidants include catalase (CAT), superoxide dismutase, and glutathione peroxidase, which can only be produced within our body and cannot be supplemented orally3. In contrast, non-enzymatic antioxidants, such as vitamin E, vitamin C, carotenoids, and glutathione (GSH), can be supplemented orally. Of these, GSH, the so-called master antioxidant, is probably the most important non-enzymatic antioxidant in our body because it is found in every single cell, thus maximizing the activities of all other antioxidants within the cellular compartment2.
In the present study, the state of oxidative stress was chemically induced using kainic acid (KA), an excitotoxic substance that causes considerable oxidative damage to the brain4, 5. KA is a naturally occurring substance, isolated and extracted from red algae (Digenea simplex), which binds to the KA1 and KA2 receptors. These receptors are a subtype of the ionotropic glutamate receptor family6, and they are highly expressed in several areas of the brain, including the hippocampus7, which explains the selective vulnerability of the hippocampus to the excitotoxic effect of KA8. Previously, KA has been used to elucidate the mechanisms underlying oxidative stress, inflammation, and apoptosis in neurodegenerative diseases9, 10.
The use of nanotechnology in food safety and biomedical sciences has been constantly increasing over the last few decades. A nanoparticle is a particle of matter that has a diameter of less than 100 nm11. Because of their size, nanoparticles can cross into the placenta, blood-brain barrier, and various types of cells, and interact with DNA. There are many types of nanoparticles, which can be classified as organic or non-organic nanoparticles, which can be synthetically produced or obtained as a byproduct (non-synthetic)12. One of the most prominent and fascinating non-organic metal nanoparticles is silver nanoparticles, which have been used in various fields, such as the pharmaceutical industry, food industry, household, and healthcare-related products, because of their distinctive physical and chemical properties. In the medical field, silver nanoparticles are used in diagnostic procedures, drug delivery, anticancer agents, and orthopedic devices13.
Green synthesis refers to methods using natural products (e.g., plant extract), which is increasingly being used to produce nanoparticles for applications in biology, medicine, and engineering. Green synthesis has many advantages over synthetic chemical or physical methods as it is non-toxic, environmentally friendly, economical, and more sustainable14. In the green synthesis methods, polyphenols and proteins contained in plant-based materials function as reducing agents to reduce metal ions into metal nanoparticles15. Under certain circumstances, metal nanoparticles synthesized using plant-based materials have a higher quality compared to similar nanoparticles synthesized using physical or synthetic chemical methods. For instance, it has been shown that metal nanoparticles (iron oxide) produced using the green synthesis routes have a much smaller average size (2 – 80 nm) compared to that of particles produced using the synthetic chemical methods (87 – 400 nm) Gokila (2021)16.
We have previously synthesized silver nanoparticles using a green synthesis method, in which Tualang honey (TH) was used as the natural raw material17. TH is a well-known local honey that contains the highest level of antioxidant content compared to other local Malaysian honey18. The antioxidant contents of TH include flavonoids, phenolic acids, ascorbic acid, and carotenoids, which are believed to be responsible for many of its health benefits19, 20. The silver nanoparticles synthesized using TH were characterized by X-ray diffraction, Fourier transform infrared spectroscopy, field emission scanning electron microscopy, and transmission electron microscopy. The synthesized silver nanoparticles were 22 nm in diameter and exhibited antioxidant activity and reducing power values of 95.54 ± 0.96% and 1032.30 ± 102.76 µm Fe(II), respectively17. Recently, it has been reported that silver nanoparticles synthesized using TH can ameliorate seizures, locomotor activity, and memory function in KA-induced status epilepticus in male rats21.
To date, it is not known whether TH-mediated silver nanoparticles (THSN) can attenuate oxidative stress in the hippocampus of the KA-induced excitotoxicity-mediated neurodegeneration model. Therefore, the present study aimed to determine the oxidant/antioxidant status of THSN by measuring the levels of malondialdehyde (MDA), total nitrate/nitrite (NOx), protein carbonyl (PCO), GSH, total antioxidant status (TAS), and CAT activity in the hippocampus of the KA-induced oxidative stress rat model.
Methods
TH procurement
TH (AgroMas®) was supplied by the Federal Agricultural Marketing Authority, Ministry of Agriculture and Agro-Based Industry, Kedah, Malaysia.
THSN preparation
The THSN was prepared using the green synthesis method17. First, TH was synthesized with silver nitrates to obtain the nanoparticle powder. The TH solution was adjusted to pH 8.0 by adding a few drops of sodium hydroxide (NaOH), before adding 0.1 M of silver nitrate solution. A color change from light brown to dark brown was observed, indicating the successful synthesis of THSN. The solution was dried overnight in the oven at 60 °C, and the nanoparticles were collected in powdered form and stored at room temperature. THSN solution was freshly prepared by dissolving the THSN powder in 0.5 mL of distilled water.
Animals
Adult male Sprague Dawley rats (weighing approximately 200 – 250 g; aged 8 – 10 weeks) were purchased from the Animal Research and Service Centre, Universiti Sains Malaysia (USM) Health Campus, Kubang Kerian, Kelantan. The animals were housed individually in polypropylene cages and maintained at a temperature of 25 ± 2 °C under a 12 h light/dark cycle. The animals were acclimatized for at least 7 days and provided rat pellets and water ad libitum. The experimental procedure was reviewed and approved by the Animal Ethic Committee of USM [USM/IACUC/2018/(111)(904)]. All experimental procedures involving animals were performed following the Institutional Guidelines for the Care and Use of Animals for Scientific Purposes.
Experimental groups
A total of 72 male rats were randomized into six major groups (n = 12 rats per major group). The major groups were established as follows:
Group 1: Control — Rats were orally administered distilled water. Thirty minutes after the last oral treatment, the rats were injected subcutaneously with 0.5 mL of saline solution.
Group 2: THSN 10 mg — Rats were orally administered THSN (10 mg/kg). Thirty minutes after the last oral treatment, the rats were injected subcutaneously with 0.5 mL of saline solution.
Group 3: THSN 50 mg — Rats were orally administered THSN (50 mg/kg). Thirty minutes after the last oral treatment, the rats were injected subcutaneously with 0.5 mL of saline solution.
Group 4: KA only — Rats were orally administered distilled water. Thirty minutes after the last oral treatment, the animals were injected subcutaneously with KA solution (15 mg/kg).
Group 5: THSN 10 mg + KA — Rats were orally administered THSN (10 mg/kg). Thirty minutes after the last oral treatment, the animals were injected subcutaneously with KA solution (15 mg/kg).
Group 6: THSN 50 mg + KA — Rats were orally administered THSN (50 mg/kg). Thirty minutes after the last oral treatment, the animals were injected subcutaneously with KA solution (15 mg/kg).
Each major group was further divided randomly into two subgroups depending on the time of sacrifice (24 h and 5 days after KA induction; n = 6 rats per subgroup). For the THSN pre-treatment, 10 and 50 mg/kg dosages were chosen for this study because of the antidiabetic effect and increased antioxidant activities reported in rat models22, 23. Each group was pre-treated five times with the respective treatments at 12 h intervals.
The dose of KA (15 mg/kg) was reported to be sufficient in eliciting demonstrable damage in different brain regions such as the cerebellum and brainstem5. Moreover, a subcutaneous injection of saline was given to animals in the control group (no KA induction). Since the KA dosage of 15 mg/kg was associated with a high mortality rate, diazepam (10 mg/kg) was injected approximately 90 min after the onset of the first generalized seizure, as a precaution, to increase the survival rate of KA-induced rats5, 9. Finally, the rat hippocampus was used for biochemical analysis, to assess oxidative stress markers.
Biochemical analysis
At the time of sacrifice (24 h or 5 days after KA induction), rats were euthanized with an overdose of sodium pentobarbital (100 mg/kg, intraperitoneal), followed by decapitation using a guillotine. First, the hippocampus from each animal was quickly extracted from the brain, isolated, weighed, and stored at −80 °C until use. Then, the tissue samples were homogenized (10% w/v) in ice-cold 0.1 M phosphate-buffered saline (pH 7.4). Next, the homogenates were centrifuged (10,000 x g) for 10 min, and the supernatants were aliquoted and stored at −80 °C until analysis.
The oxidative stress parameters were determined based on the levels of MDA, NOx, PCO, GSH, TAS, and CAT activity. These markers were determined using commercially available ELISA kits (Qayee, Wuhan) via the double antibody enzyme-linked immunosorbent one-step process. The assays were performed following the manufacturer’s instructions.
Statistically analysis
The results were analyzed using SPSS software version 26 (SPSS Statistics, IBM, Chicago, USA). The datasets were subjected to normality and homogeneity of variance analysis using Levene’s test. The normally distributed data and equal variance were analyzed using a parametric test, a one-way analysis of variance followed by Tukey’s post hoc test, to determine the mean differences in all parameters between the groups. The data were expressed as the mean ± standard error of the mean and considered significant when the p-value was less than 0.05.

Level of MDA in the hippocampus of KA-induced rats. The results were expressed as mean ± SEM. The significant difference was determined by parametric test; one way ANOVA followed by Tukey post-hoc test with p < 0.05 indicates statistically difference. * p < 0.05 versus KA only group; ** p < 0.01 versus KA only group.

Level of NOx in the hippocampus of KA-induced rats. The results were expressed as mean ± SEM. The significant difference was determined by parametric test; one way ANOVA followed by Tukey post-hoc test with p < 0.05 indicates statistically difference. * p < 0.05 versus KA only group; ** p < 0.01 versus KA only group.
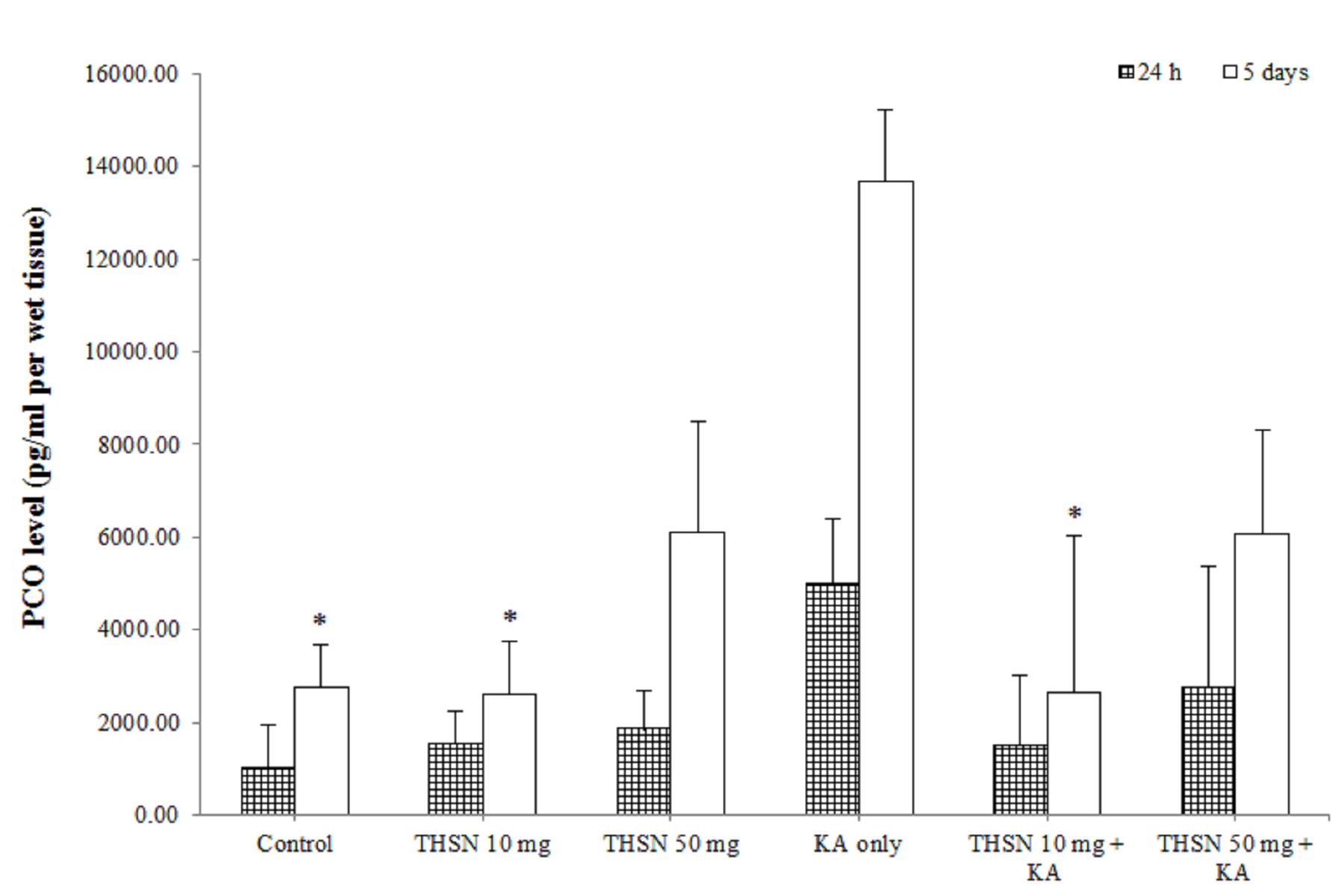
Level of PCO in the hippocampus of KA-induced rats. The results were expressed as mean ± SEM. The significant difference was determined by parametric test; one way ANOVA followed by Tukey post-hoc test with p < 0.05 indicates statistically difference. * p < 0.05 versus KA only group.

Activity of CAT in the hippocampus of KA-induced rats. The results were expressed as mean ± SEM. The significant difference was determined by parametric test; one way ANOVA followed by Tukey post-hoc test with p < 0.05 indicates statistically difference. * p < 0.05 versus KA only group; ** p < 0.01 versus KA only group; *** p < 0.001 versus KA only group.
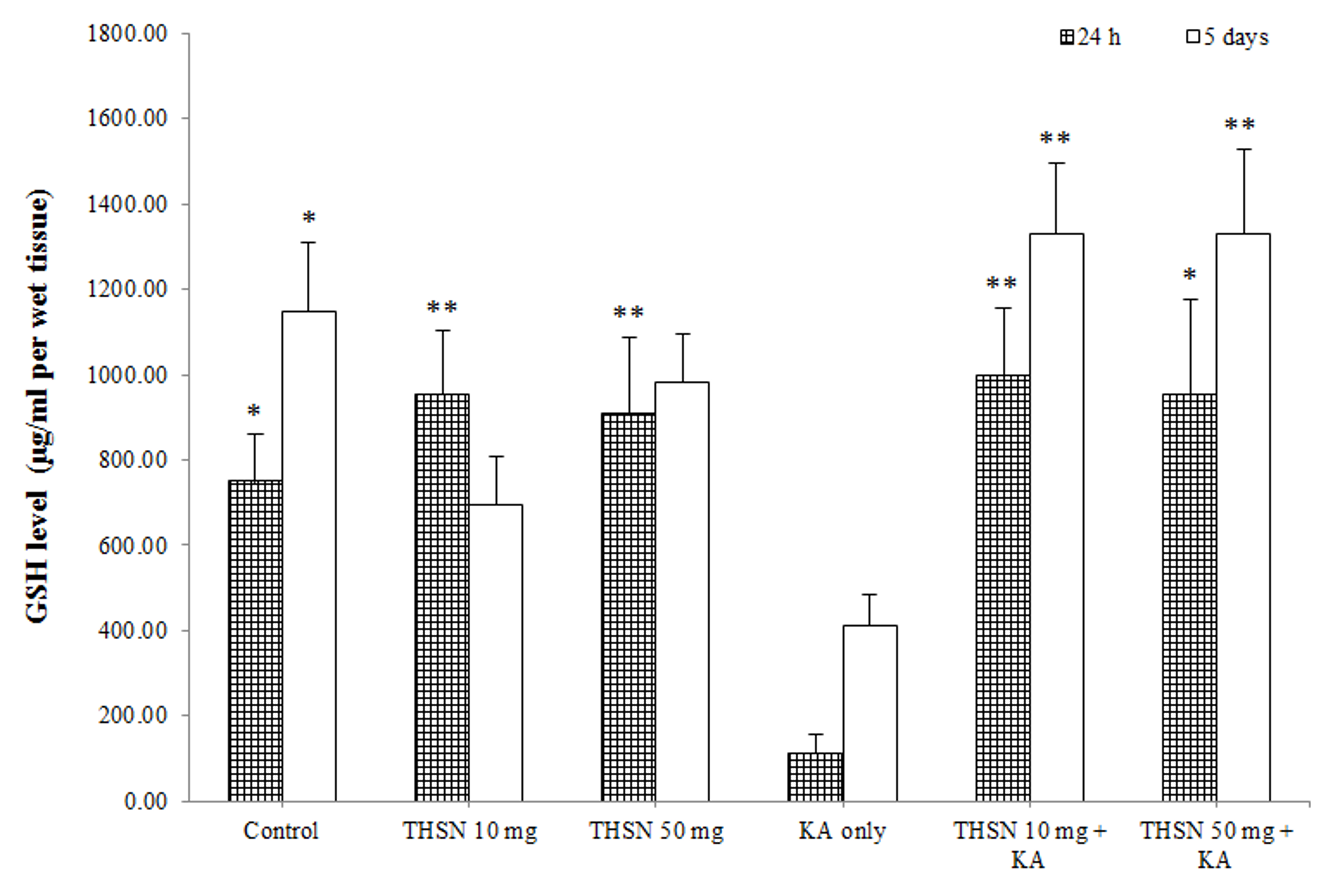
Level of GSH in the hippocampus of KA-induced rats. The results were expressed as mean ± SEM. The significant difference was determined by parametric test; one way ANOVA followed by Tukey post-hoc test with p < 0.05 indicates statistically difference. * p < 0.05 versus KA only group; ** p < 0.01 versus KA only group.
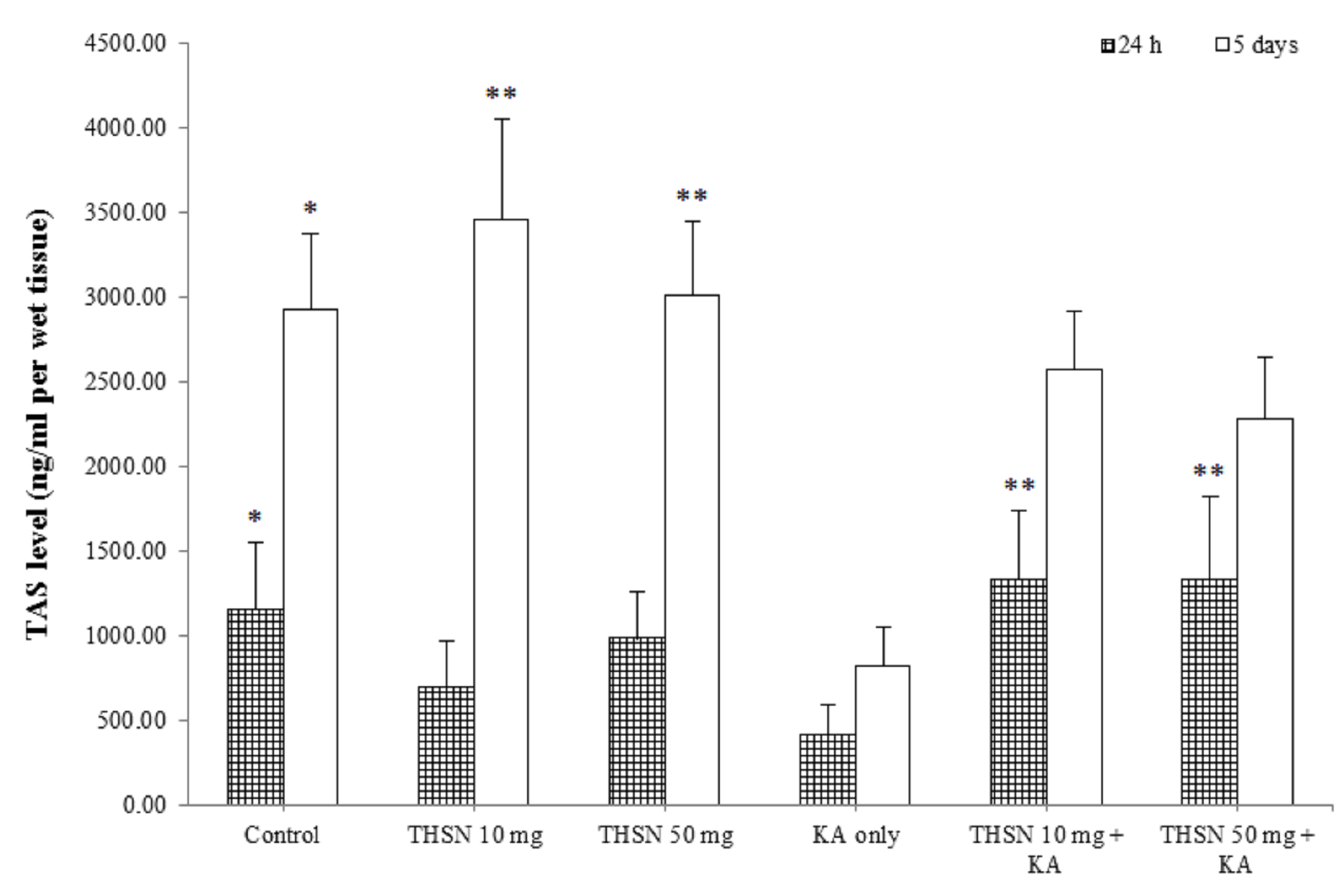
Level of TAS in the hippocampus of KA-induced rats. The results were expressed as mean ± SEM. The significant difference was determined by parametric test; one way ANOVA followed by Tukey post-hoc test with p < 0.05 indicates statistically difference. * p < 0.05 versus KA only group; ** p < 0.01 versus KA only group.
Results
Malondialdehyde (MDA) level
The present study showed that there were significant differences in MDA levels among rats in the 24 h subgroups [F (5, 30) = 4.099, p < 0.01] (p = 0.006). The level of MDA in the KA-only group was significantly higher than control (p < 0.01) and THSN 10 mg (p < 0.05) groups (Figure 1). There were significant reductions in the MDA levels in the THSN 10 mg + KA and THSN 50 mg + KA groups compared to the KA-only group, suggesting an improvement in lipid peroxidation following THSN pre-treatment. Moreover, in the 5 days subgroups, a decreasing trend of the MDA level was observed in the THSN pre-treatment groups; however, there was no significant difference (p > 0.05) between the groups, except for the control group (p < 0.05), when compared with KA only (Figure 1).
Total nitrate/nitrite (NOx) level
In the present study, there was a significant difference in NOx levels among the 24 h subgroups [F (5, 30) = 5.307, p < 0.01] (p = 0.001). The level of NOx in the KA-only group was significantly higher compared to the control (p < 0.05), THSN 10 mg (p < 0.01), and THSN 50 mg (p < 0.01) groups. In addition, the NOx levels were significantly lower in the THSN 10 mg + KA (p < 0.01) and THSN 50 mg + KA (p < 0.01) groups compared to the KA-only group (Figure 2). These results demonstrated that administration of THSN can prevent KA-induced elevation of NOx levels in the hippocampus at 24 h after KA induction. Moreover, in 5 days subgroups, there was no significant difference (p > 0.05) in NOx levels between all 5 days subgroups, although a decreasing trend was observed following THSN pre-treatment, in comparison to the KA-only group. These results demonstrated that administration of 10 mg of THSN can prevent KA-induced elevation of NOx levels at 24 h, but not 5 days, after KA induction (Figure 2).
Protein carbonyl (PCO) level
In the present study, the result showed that there was no significant difference (p > 0.05) in PCO levels between all groups at 24 h after KA induction (Figure 3). However, in 5 days subgroups, there were significant differences in the hippocampal PCO levels between the groups [F (5, 30) = 3.587, p < 0.05] (p = 0.012). There was a significant increase in PCO levels in the KA-only group compared to the control (p < 0.05) and THSN 10 mg (p < 0.05) groups. There was also a significant reduction in hippocampal PCO levels in the THSN 10 mg + KA group (p < 0.05) compared to the KA-only group (Figure 3). These results demonstrated that administering 10 mg of THSN can prevent KA-induced elevation of PCO levels in the hippocampus at 5 days, but not at 24 h after KA induction.
Catalase (CAT) activity
There was a significant difference in the hippocampal CAT activity between the groups at 24 h [F (5, 30) = 9.277, p < 0.001] (p = 0.000). There was a significant decrease in CAT activity in the KA-only group compared to the control (p < 0.01), THSN 10 mg (p < 0.01), and THSN 50 mg (p < 0.001) groups. In addition, CAT activity was significantly increased in the THSN 50 mg + KA group (p < 0.05) compared to the KA-only group (Figure 4). Moreover, in 5 days subgroups, there was a significant reduction in CAT activity in the KA-only group compared with the control (p < 0.01), THSN 10 mg (p < 0.01), and THSN 50 mg (p < 0.05) groups. Additionally, the CAT activity was significantly increased in the THSN 10 mg + KA group (p < 0.001) compared to the KA-only group (Figure 4). These results suggested that pre-treatment with THSN 10 mg and THSN 50 mg prevented the KA-induced reduction of CAT activity after 24 h and 5 days, respectively.
Reduced glutathione (GSH) level
There was a significant difference in the GSH levels among the 24 h subgroups [F (5, 30) = 5.245, p < 0.01] (p = 0.002). The post hoc test showed that the GSH level in the KA-only group was significantly lower than that in the control (p < 0.05), THSN 10 mg (p < 0.01), and THSN 50 mg (p < 0.01) groups. Interestingly, GSH levels in the THSN 10 mg + KA (p < 0.01) and THSN 50 mg + KA (p < 0.05) groups were significantly higher than that in the KA-only group (Figure 5). Meanwhile, the 5 days subgroups also demonstrated significant differences [F (5, 30) = 6.528, p < 0.001] (p = 0.000). The GSH level was significantly reduced in the KA-only group compared to the control (p < 0.05). However, GSH levels were significantly higher in the THSN 10 mg + KA (p < 0.01) and THSN 50 mg + KA (p < 0.01) groups compared to the KA-only group (Figure 5). These results demonstrated that the administration of 10 mg or 50 mg of THSN was effective to prevent KA-induced depletion of GSH in the hippocampus.
Total antioxidant status (TAS) level
The present study demonstrated a significant difference in TAS levels among the 24 h subgroups [F (5, 30) = 6.528, p = 0.000]. The post hoc test showed that the TAS level in the KA-only group was significantly lower compared to the control (p < 0.05). The TAS levels were significantly higher in the THSN 10 mg + KA (p < 0.01) and THSN 50 mg + KA (p < 0.01) groups compared to the KA-only group (Figure 6). Moreover, the TAS levels were significantly different [F (5, 30) = 4.858, p < 0.01] (p = 0.002) among the 5 days subgroups. The TAS level in the KA-only group was significantly decreased compared to the control (p < 0.05), THSN 10 mg (p < 0.01), and THSN 50 mg (p < 0.01) groups (Figure 6). However, there were no significant differences between THSN 10 mg + KA and THSN 50 mg + KA groups compared to the KA-only group. These results demonstrated that administering 10 mg and 50 mg of THSN are effective to prevent KA-induced reduction of TAS level in the hippocampus at 24 h, but not at 5 days after KA induction.
Discussion
The current study investigated the state of oxidative stress in the hippocampus by evaluating MDA, PCO, and NOx levels. MDA is widely used as a convenient biomarker for lipid peroxidation of polyunsaturated fatty acids because it readily reacts with thiobarbituric acid24. In the present study, MDA levels were significantly elevated at 24 h and 5 days following KA administration, indicating that a significant level of lipid peroxidation occurred in the hippocampus. This finding is consistent with other studies25, 26.
Another important lipid peroxidation biomarker is NOx, a messenger molecule in the central nervous system27. A high level of NOx has been associated with a rise in the highly reactive toxic peroxynitrite radical, which may cause cellular damage28. It has been demonstrated that glutamate-KA receptor stimulation induces neuronal NO release, which in turn modulates glutamate transmission29. The current study demonstrated that NOx levels in the rats’ hippocampus were significantly elevated at 24 h after KA administration, but not 5 days after. This time-dependent outcome was consistent with an earlier report, where KA significantly increased NOx levels in the right temporal lobe as early as 60 to 90 min after KA induction, and the NOx levels decreased gradually after 120 min30.
In the present study, the occurrence of protein oxidation was demonstrated by the raised PCO levels at 24 h after KA administration. Protein oxidation involves the covalent modification of amino acid side chains and protein backbones, which results in protein fragmentation or protein-protein cross-linking, altering their conformation, solubility, and enzyme activities31. The use of PCO as a biomarker of oxidative stress has several advantages because of its stability and relatively early formation. The result of the present study is similar to previous studies, which reported KA-induced protein oxidation in different regions of the brain, including the hippocampus5, 32. The current study also demonstrated that PCO levels were remarkably increased at 5 days after KA administration but not at 24 h. Furthermore, Dutra (2018) demonstrated that rats in the epilepsy animal model exhibited higher levels of protein carbonyl in the hippocampus during the early silent phase (3 – 5 days after status epilepticus)33. An elevated PCO level may be caused by excessive protein oxidation or the decreased capacity of an organism to eliminate oxidatively damaged proteins.
The hippocampus is susceptible to oxidative stress because of the high levels of serotonin that promote free radical formation34, high content of polyunsaturated fatty acids and iron, and low antioxidant defenses such as CAT and GSH35. Consistent with previous reports, systemic KA administration affected CAT activity in the brain36, 37. We observed that the CAT activity in the hippocampal tissue was significantly lowered in the KA-induced rats at 24 h and 5 days, which represents an important index of oxidative stress. The present study agrees with Liu (2015), where KA induction led to lower CAT activity in the hippocampus38.
The current study also demonstrated the presence of low GSH levels in the hippocampus at 24 h and 5 days after KA induction, indicating a low GSH antioxidant defense mechanism. GSH is the most abundant intracellular thiol in the brain39, 40, which maintains the redox balance of cells, reduces oxidized particles, and detoxifies reactive oxygen species41. Therefore, the amount of GSH may be a valuable indicator of oxidative stress levels.
The use of exogenous antioxidants to inhibit the production of free radicals is well-recognized in the literature38. In the present study, pre-treatment with THSN prevented the KA-induced increase in MDA, NOx, and PCO while simultaneously enhancing the production of CAT, GSH, and TAS at different time points, which confirms the protective effects of THSN against oxidative stress. The pre-treatments with THSN significantly reduced lipid peroxidation, NOx, and TAS after 24 h and protein oxidation after 5 days of KA administration, indicating the protective effects of these substances against oxidative stress on membrane lipids and cellular proteins. However, THSN did not significantly increase the TAS level or reduce the MDA or NOx levels after 5 days, possibly because of a lower rate of bioavailability, rapid metabolism, and elimination of active ingredients in THSN42, 43.
The increase in GSH and TAS levels by THSN suggested antioxidant properties of THSN possibly by increasing the brain’s endogenous defense. TAS is used to measure the overall antioxidant status of the body, which helps elucidate the protective effect displayed by antioxidants, reflecting their improvement in endogenous defense44. The findings in this study showed that 10 mg and 50 mg of THSN at 24 h after KA administration significantly improved TAS levels in the hippocampus.
The neuroprotective effects of THSN are not surprising because THSN exhibited remarkable antioxidant activity17. Previous studies have demonstrated that the majority of silver nanoparticles synthesized using green methods, particularly plant extracts, have free radical scavenging activity45. The THSN synthesized in the current study contains alcohols, phenols, amide, carboxylate ions, and protein, and exhibited high antioxidant activity, as described previously17. Moreover, TH, the reducing agent used to synthesize the silver nanoparticles, is known to contain antioxidants, such as phenols and flavonoids, with a high total phenolic content (251.7 ± 7.9 mg gallic acid/kg honey), high total antioxidant activity (322.1 ± 9.7 μM Fe(II)), and good antiradical activity (41.30 ± 0.78% inhibition)46.
The attachment of antioxidant molecules (such as phenols and flavonoids) to the surfaces of nanoparticles may create phytoantioxidant-functionalized nanoparticles that can synergistically protect against oxidative damage45. These phytoantioxidant-functionalized nanoparticles are a safer alternative to nanoparticles produced using physical or synthetic chemical methods. Creating nanoparticles using natural antioxidants, like honey, is advantageous because it can increase stability and biocompatibility while diminishing toxicity, in addition to preserving the desirable properties of natural compounds.
Taken together, the present study supports earlier findings showing that KA administration involves oxidative stress pathways5, 47, 48. KA-induced oxidative stress is associated with enhanced lipid peroxidation and protein oxidation while reducing both the enzymatic and non-enzymatic anti-oxidative defenses of the body. Previously, it has been shown that KA-induced oxidative stress is associated with a rise in extracellular glutamate levels in the hippocampus, which is associated with free radicals generation and a reduction in residual antioxidant activity10. Notably, the present study demonstrated that these KA-induced changes can be improved by introducing silver nanoparticles produced using TH, which are non-toxic, environmentally friendly, and economically more sustainable.
Conclusions
The present study concluded that THSN attenuates oxidative stress in the hippocampus of KA-induced rats. THSN has been shown to prevent the KA-induced increase in oxidative stress markers and simultaneously improve the anti-oxidative defenses. Further investigations at the molecular level of the mechanism may further clarify the protective effects of THSN against KA-induced oxidative stress in rats.
Abbreviations
CAT: Catalase, ELISA: enzyme-linked immunosorbent assay, GSH: Reduced glutathione, KA: Kainic acid, MDA: Malondialdehyde, NOx: total nitrate/nitrite, PCO: Protein carbonyl, SEM: Standard error mean, SPSS: Statistical package for social sciences, TAS: total antioxidant status, TH: Tualang honey, THSN: Tualang honey-mediated silver nanoparticles
Acknowledgments
The authors gratefully acknowledge Universiti Malaysia Kelantan, Animal Research and Service Centre (ARASC), and Central Research Laboratory (CRL), Unversiti Sains Malaysia, Health Campus for providing the lab facilities for this research work.
Author’s contributions
SKNS developed the original idea and designed the study. PVR gave advices regarding on preparation of THSN. SKNS and SM assist the biochemical study. MAA and SKNS supervised the work. HH prepared the THSN, performed the experiment, analysed the results and drafted the article. All authors have read, revised and approved the article submission.
Funding
This research was financially supported by the Universiti Sains Malaysia under Research University (Individual) (RUI Grant No: 1001/PPSP/8012249).
Availability of data and materials
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Ethics approval
The experimental procedure was approved by the Animal Ethic Committee of USM [USM/IACUC/2018/(111)(904)]. All experimental and procedures involving animals were performed following the Institutional Guidelines for the Care and Use of Animals for Scientific Purposes. No human volunteers were used.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

