The role of inflammation and oxidative stress markers in the occurrence and severity of coronary artery disease in young patients with ST-segment elevation myocardial infarction
- Medical Biochemistry Department, Faculty of Medicine, Assiut University, Assiut, Egypt
- Assiut International Center of Nanomedicine, Al-Rajhy Liver Hospital, Assiut University, Assiut, Egypt
- Biochemistry Department, Faculty of Pharmacy, Sphinx University, New Assiut, Egypt
- Cardiology Department, Faculty of Medicine, Assiut University, Assiut, Egypt
Abstract
Background: Atherosclerosis is modulated by inflammation and oxidative stress, which play pivotal roles in the pathogenesis of coronary artery disease (CAD), especially in young patients without traditional risk factors for atherosclerosis. In this study, we aimed to study the role of some inflammatory and oxidative stress markers in triggering ST segment elevation myocardial infarction in Egyptian young patients and the potential correlation to the severity of coronary artery lesions in the Egyptian population.
Methods: This case-control study recruited 115 premature STEMI patients (aged <45 years) and 55 age-matched healthy controls. Serum CRP, TNF-a, IL-10, and oxidative stress biomarkers [reduced glutathione, total antioxidants, L-ascorbic acid, nitric oxide (NO), and lipid peroxides] were assayed using commercially available kits and correlated with the coronary artery lesion severity, assessed by the SYNTAX score (SS). The ROC curve assessed the biomarker potential of CRP, TNF-a, IL-10, and the TNF-a/IL-10 ratio to discriminate patients from healthy controls.
Results: In our study, we found that serum CRP (38.7 ± 1.9 versus 0.8 ± 0.04 mg/L; P < 0.001), TNF-a (68.4 ± 4.7 pg/mL versus 10.8 ± 0.7 pg/mL; P < 0.001), IL-10 (11.9 ± 0.8 pg/mL versus 8.7 ± 0.4 pg/mL; P = 0.001), and TNF-a/IL-10 ratio (6.8 ± 0.5 versus 1.7 ± 0.2; P < 0.001) were significantly higher in patients compared to healthy controls. Regarding oxidative stress markers, serum T-AOC levels (25.5 ± 0.9 versus 14.9 ± 0.4 U/mL; P < 0.001), NO (10.5 ± 0.3 versus 8.1 ± 0.3 nmol/mL; P < 0.001), serum LPO levels (15.8 ± 0.4 versus 4.1 ±0.2 mmol/L; P < 0.001) and lower GSH levels (3.8 ± 0.1 mg/mL versus 5.2 ± 0.2; P < 0.001) were significantly different in patients versus controls. CRP, TNF-a, and NO levels have been significantly correlated with the severity of CAD as assessed by the SYNTAX score.
Conclusion: Inflammation and oxidative stress are pathogenically implicated in premature STEMI and correlated with the severity of CAD lesions. The investigated biomarkers can be utilized in risk stratification and are theranostically targetable.
Introduction
Coronary artery disease (CAD) is a primary cause of death worldwide, with an increasing prevalence1. The bigger challenge is the premature CAD affecting young populations, which is an unanticipated risk with a high recurrence rate and socio-economic impact2. Premature CAD is more prevalent among men who are obese, smokers, and those with dyslipidemia, a sedentary lifestyle, and drug abuse. Although they have a lower rate of hypertension and diabetes, their traditional cardiovascular risk factors are mostly uncontrolled, owing to their low adherence to risk factor modification measures3, 4.
Atherosclerotic cardiovascular disease is characterized by chronic inflammation, on top of being a lipid metabolic disorder. Inflammation heralds the initiation, progression, instability, and rupture of the atherosclerotic plaque5, 6. C-reactive protein (CRP), the homopentameric protein, is principally secreted from hepatocytes, macrophages, endothelial cells, lymphocytes, smooth muscle cells, and adipocytes. CRP is an acute systemic inflammatory biomarker that spikes in reaction to infection or inflammatory disorders, like cardiovascular disease, in asymptomatic people. CRP levels above 3 mg/L are pathogenic and associated with the severity and prognosis of atherosclerotic CAD7, 8.
Tumor necrosis factor (TNF)-α is a pro-inflammatory pleiotropic key regulatory cytokine implicated in the pathophysiological mechanisms that govern inflammation9. The TNF-α role is paramount in the initiation stage of the formation of the atherosclerotic plaque, as it prevents the clearance of damaged vascular cells and apoptotic bodies10. In contrast, the anti-inflammatory interleukin (IL)-10 is crucial in counteracting inflammation by controlling the homeostasis of inflammatory cells11. Moreover, oxidative stress is associated with coronary atherosclerosis and the reduced bioavailability of the angio-protective nitric oxide (NO) due to its conversion into the more damaging peroxynitrite radical12. Inflammation, gauged with CRP and governed by the interplay among oxidative stress, TNF-α, and IL-10, is implicated in coronary atherosclerosis and is related to its severity. They are being investigated as theranostic targets13, 14. Uneven or disrupted blood flow can lead to the development of atherosclerotic plaque. Low wall shear stress (WSS), produced by this flow pattern (which is a well-known mechanism of atherosclerosis), can cause arterial inflammation and propel the development of atherosclerosis and plaque15. Adhesion proteins and other inflammatory chemicals are produced in greater quantities when WSS is reduced, which attracts leucocytes and guides their migration into the artery wall15. This mechanism could elucidate how local inflammation events that are far from atherosclerotic lesions may trigger inflammatory activation and the advancement of coronary plaque15.
In this study, we planned to investigate how CRP, TNF-α, IL-10, and oxidative stress are implicated in the development of premature ST segment elevation myocardial infarction (STEMI), along with the correlation to disease severity in young Egyptian patients, based on their major role as pathogenic mediators and severity biomarkers.
Methods
Setting and participants
This prospective case-control study involved 115 consecutively enrolled and consented young CAD patients who presented with acute STEMI. Inclusion criteria: acute STEMI patients (aged < 45 years); for the disease diagnosis, we employed the European Society of Cardiology guidelines and its 4 MI definition16. The study was conducted over six months, from April 2022 to September 2022. The study sample size was calculated with a power of 0.95 and a hypothetical effect size of 0.33, using G Power software. Patients were admitted to the Department of Cardiology, Assiut University Hospitals, Assiut, Egypt. As a control group, 55 age-matched and apparently healthy volunteers were recruited. We excluded: STEMI patients over 45 years, those with chronic diseases (diabetes, hepatic or renal failure), COVID-19 patients, and addicts (for drugs and/or alcohol). The patient group is part of our previous cohort study that investigated the implication of PCSK-9 in premature CAD pathogenesis17.
Ethical considerations
The study was carried out in compliance with the Helsinki Declaration principles after being approved by the Institutional Review Board, Faculty of Medicine, Assiut University (Approval#: IRB17200689). Volunteering participants signed the informed consent form after being briefed on the nature of the study and their roles and rights.
Assessment of clinical and laboratory parameters
After recording the clinical data, the socio-demographic and anthropometric characteristics of the participants, the presence of traditional risk factors (family history, smoking, hypertension, dyslipidemia, and body mass index [BMI; kg/m²]), and the relevant routine laboratory findings were retrieved. On admission, resting 12-lead surface ECG recording, echocardiography, and percutaneous coronary intervention (primary PCI) were conducted. All coronary lesions with >50% diameter stenosis in a vessel >1.5 mm in diameter were assessed by interventional cardiologists for each patient to determine the SYNTAX score (SS), based on the SS calculator18. According to SS, the disease severity was stratified into three levels: SS ≤ 12, >12–≤21.5, and >21.519. Healthy volunteers were subjected to history taking, physical examination, and assessment of their BMI.
Serum recovery and laboratory biomarker investigations
After admission, 5 mL of peripheral venous blood was collected from each participant in a plain tube, left to clot, centrifuged at 3000 rpm for 10 minutes, and the separated serum was stored at -20 °C. We used commercially available specific quantitative ELISA kits to assess serum protein biomarkers: 1) Cardiac troponin T (cTnT) and creatine phosphokinase-MB (CK-MB) (Cat.# ab223860 and ab193696; Abcam, UK); and, 2) CRP (with 0.44 pg/mL sensitivity), TNF-α, and IL-10 (Cat.# EL0036Hu, SL1761Hu, and SL0967Hu, Sunlong Biotech Co., Hangzhou, China), as instructed by the manufacturer. We used quantitative colorimetric assay kits (Spectrum, Cairo, Egypt) to assess lipid profile and relevant organ functions [aspartate aminotransferase (AST; REF: 260-002), alanine aminotransferase (ALT; REF: 264-002), alkaline phosphatase (ALP; REF: 216-001), creatine phosphokinase (CPK; REF: 238-002), creatinine (REF: 235-001), urea/BUN (REF: 319-001), total cholesterol (REF: 230006), high-density lipoprotein cholesterol (HDL-C; REF: 266003), triglycerides (REF: 314005), and bilirubin (total and direct; REF 225-001), as instructed]. We calculated LDL-C to be = Total Cholesterol–Triglycerides/5–HDL-C. Total anti-oxidative capacity (TAC; U/mL) measured by Fe³+-2,3,6-tri (2-pyridyl)-1,3,5-triazine reagent, and lipid peroxidation (LPO; μmol/L) measured as malondialdehyde (MDA), after the reaction with thiobarbituric acid, were colorimetrically assayed (Cat# AK0455-100T-96S and AK0694-50T-58S, Sunlong Biotech Co., Hangzhou, China). Serum nitric oxide (NO; nmol/mL) was estimated as nitrite using Griess reagent against the sodium nitrite standard20. Serum reduced glutathione (GSH; μg/mL) concentration was estimated by the DTNB reagent21, 22. Estimation of serum L-ascorbic acid (μg/mL) was done using Folin phenol reagent vs. L-ascorbic acid standard23.
Statistical analysis
The nominal data were expressed as frequency (percentage), and Chi²-test was used to compare them between groups. The continuous data were expressed as mean and standard error (SE), and the Student’s t-test or one-way analysis of variance (ANOVA), with Bonferroni post hoc test, was used to compare their means among groups. Receiver Operating Characteristic (ROC) curve analysis was used to determine the area under the curve (AUC) for each of CRP, TNF-α, and IL-10 to determine their sensitivity and specificity to discriminate patients from healthy controls at several cutoff points. A probability (P value) of <0.05, at a confidence level of 95%, was considered statistically significant. Data entry, analysis, and presentation were done using the Statistical Package for the Social Sciences (SPSS) version 20 (IBM, Armonk, New York, USA).
Baseline characteristics and routine serum laboratory workup of the patients with premature ST segment elevation myocardial infarction (STEMI) patients (n = 115) compared to the healthy control group (n = 55)
|
|
Patients (n=115) |
Control (n=55) |
P-value |
|
Age, year |
38 ± 0.4 |
38 ± 0.3 |
0.63 |
|
BMI, kg/m2 |
26 ± 0.4 |
24 ± 0.2 |
> 0.001 |
|
Sex |
Male: 108, (94.7%) Female: 6, (5.3%) |
Male: 50, (90.9%) Female: 5, (9.1%) |
0.66 |
|
Smoking |
Smokers: 92, (80%) |
Smokers: 6, (10.9%) |
> 0.001 |
|
Hypertention |
Hypertensive: 20, (17.3%) |
Hypertensive ( 0 ) |
0.14 |
|
Family history of PCAD |
Yes: 19, (16.5%) |
Yes: 2, (3.6%) |
0.19 |
|
Family history of hyperlipidimia |
Yes: 15, (13%) |
Yes: 4, (7.2%) |
0.73 |
|
History of previous PCI |
Yes: 11, (9.5%) |
Yes ( 0 ) |
0.20 |
|
Main CBC parameters | |||
|
WBCs, 109/µL |
10.2 ± 0.3 |
5.5 ± 0.1 |
> 0.001 |
|
Platelets, 109/L |
276.7± 7.8 |
380.2 ± 4.0 |
> 0.001 |
|
Hb, g/L |
144 ± 16 |
142 ± 18 |
0.32 |
|
Liver biomarkers | |||
|
Total bilirubin, µmol/L |
6.5 ± 0.1 |
6.3 ± 0.2 |
0.56 |
|
Direct bilirubin, µmol/L |
2.2 ± 0.07 |
2.1 ± 0.1 |
0.63 |
|
AST, µKat/L |
0.31 ± 0.01 |
0.31 ± 0.01 |
0.87 |
|
ALT, µKat/L |
0.29 ± 0.01 |
0.28 ± 0.01 |
0.63 |
|
ALP, µKat/L |
1.05 ± 0.02 |
1.06 ± 0.04 |
0.84 |
|
Kidney biomarkers | |||
|
Creatinie, µmol/L |
68.3 ± 1.4 |
55.4 ± 1.3 |
0.41 |
|
BUN, mmol/L |
6.0 ± 0.4 |
3.4 ± 0.1 |
> 0.001 |
|
Lipid profile | |||
|
LDL-C, mmol/L |
3.2 ± 0.08 |
3.1 ± 0.1 |
0.44 |
|
Total cholesterol, mmol/L |
4.8 ± 0.1 |
4.5 ± 0.1 |
0.14 |
|
HDL-C, mmol/L |
1.1 ± 0.02 |
1.5 ± 0.03 |
> 0.001 |
|
Triglycerides, mmol/L |
3.5 ± 0.3 |
3.0 ± 0.2 |
=0.04 |
|
Cardiac biomarkers | |||
|
c-Tnt, µg/L |
82.5 ± 16.4 |
0.02 ± 0.01 |
> 0.001 |
|
CK-MB, µg/L |
1118.5 ± 83.3 |
2.1 ± 0.08 |
> 0.001 |
|
CPK, µKat/L |
21.9 ± 1.7 |
0.9 ± 0.03 |
> 0.001 |
Results
Baseline characteristics and routine serum biomarkers of the premature STEMI patients and healthy controls
The age comparison of our participants was not significantly different (38 ± 0.3 years for healthy controls versus 38 ± 0.4 years for patients). BMI was significantly higher among patients compared to controls (26 ± 0.4 versus 24 ± 0.2 kg/m²; P > 0.001). While the majority of our patients were smokers, smokers constituted a minority among controls (80% versus 10.9%; P > 0.001). Hypertensive patients constituted 16.5%, and for family history, 13% had hyperlipidemia and 16.5% had premature CAD in their families. Percutaneous coronary intervention (PCI) was performed on 9.5% of our patients (
The patient group demonstrated non-significantly higher serum levels of total cholesterol (4.8 ± 0.1 mmol/L) and LDL-C (3.2 ± 0.08 mmol/L) compared to healthy controls (4.5 ± 0.1 mmol/L and 3.1 ± 0.1 mmol/L, respectively). Healthy controls had significantly greater HDL-C levels (1.5 ± 0.03 mmol/L versus 1.1 ± 0.02 mmol/L; P > 0.001) and lower triglyceride levels (3.0 ± 0.2 mmol/L versus 3.5 ± 0.3 mmol/L; P = 0.04) compared to the premature STEMI patients. Significantly high levels of CPK, CK-BM, and cTnT were observed in patients compared to healthy controls (P > 0.001) (
Changes in serum inflammatory biomarkers in the patients with premature ST-segment elevation myocardial infarction (STEMI) compared to the healthy controls
|
|
Patients (n = 115) |
Control (n = 55) |
P-value |
|
CRP mg/L |
38.7 ± 1.9 |
0.8 ± 0.04 |
> 0.001 |
|
TNF-α pg/mL |
68.4 ± 4.7 |
10.8 ± 0.7 |
> 0.001 |
|
IL-10 pg/mL |
11.9 ± 0.8 |
8.7 ± 0.4 |
=0.001 |
|
TNF-α/IL-10 |
6.8 ± 0.5 |
1.7 ± 0.2 |
> 0.001 |

Correlation between C-reactive protein (CRP) and tumor necrosis factor-α (TNF-α) among patients with premature ST segment elevation myocardial infarction (STEMI).
Changes in serum inflammatory biomarkers in premature STEMI patients versus the control group
Serum CRP levels were significantly higher in premature STEMI patients than healthy controls (38.7 ± 1.9 versus 0.8 ± 0.04 mg/L; P > 0.001). Serum levels of TNF-α (68.4 ± 4.7 pg/mL versus 10.8 ± 0.7 pg/mL; P > 0.001) and IL-10 (11.9 ± 0.8 pg/mL versus 8.7 ± 0.4 pg/mL; P = 0.001) were significantly higher in patients compared to healthy controls. The TNF-α/IL-10 ratio was significantly higher in premature STEMI patients than controls (6.8 ± 0.5 versus 1.7 ± 0.2; P > 0.001) (
Changes in serum oxidative stress parameters in the patients with premature ST-segment elevation myocardial infarction (STEMI) compared to the healthy controls
|
Parameters |
Patients (n = 115) |
Control (n = 55) |
P-value |
|
T-AOC, U/mL |
25.5 ± 0.9 |
14.9 ± 0.4 |
> 0.001 |
|
NO, nmole/mL |
10.5 ± 0.3 |
8.1 ± 0.3 |
> 0.001 |
|
GSH, µg/mL |
3.8 ± 0.1 |
5.2 ± 0.2 |
> 0.001 |
|
L-ascorbic acid, µg/mL |
7.8 ± 0.3 |
7.3 ± 0.3 |
0.172 |
|
LPO, µmole/L |
15.8 ± 0.4 |
4.1 ± 0.2 |
> 0.001 |
The association between serum inflammatory and oxidative stress biomarkers and the severity of the premature ST-segment elevation myocardial infarction (STEMI) assessed by SYNTAX score (SS)
|
Parameter |
SS ≤ 12 |
12 < SS ≤ 21.5 |
SS > 21.5 |
p-value |
|
CRP (mg/L) |
30.6 ± 2.5 |
46.3 ± 2.9 |
53.7 ± 9 |
> 0.001 |
|
TNF-α (pg/mL) |
49.3 ± 4.3 |
87.3 ± 8.6 |
88.6 ± 17.4 |
> 0.001 |
|
IL-10 (pg/mL) |
11.9 ± 1.5 |
11.7 ± 0.5 |
14.9 ± 4.4 |
0.80 |
|
TNF-α/IL-10 |
5.9 ± 0.7 |
7.6 ± 0.7 |
6.5 ± 0.9 |
0.23 |
|
T-AOC (U/mL) |
26.0 ± 1.2 |
25.8 ± 1.5 |
26.2 ± 6.3 |
0.99 |
|
NO (nmole/mL) |
9.8 ± 0.5 |
11.0 ± 0.5 |
13.5 ± 1.1 |
=0.04 |
|
GSH (µg/mL) |
3.8 ± 0.2 |
4.0 ± 0.2 |
4.3 ± 0.7 |
0.70 |
|
L-ascorbic acid (µg/mL) |
7.3 ± 0.5 |
8.8 ± 0.6 |
6.2 ± 1.7 |
0.09 |
|
LPO (µmole/L) |
15.7 ± 0.5 |
16.3 ± 0.7 |
16.5 ± 0.7 |
0.08 |
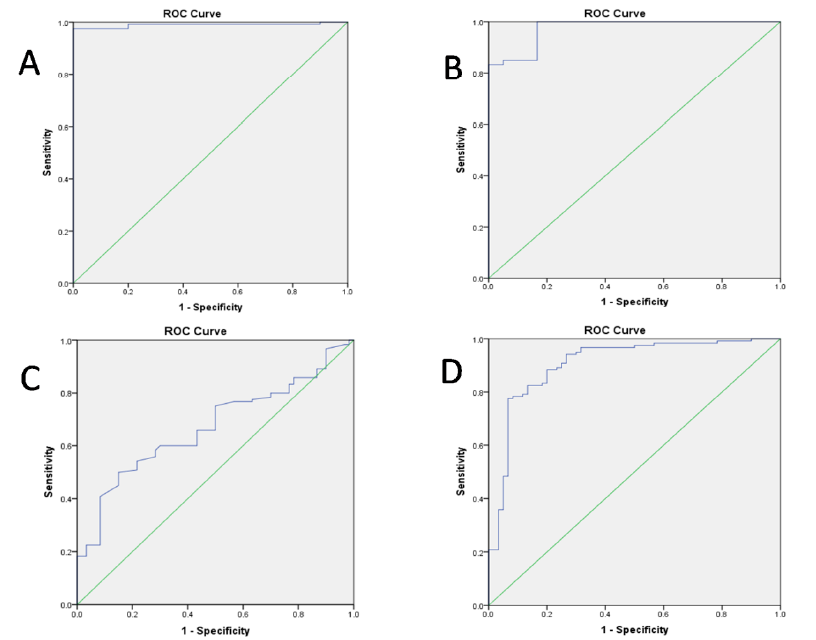
Receiver operating characteristic (ROC) analysis of serum levels of the biomarkers
|
Biomarker |
Cut off point |
Sensitivity |
Specificity |
AUC |
|
CRP |
> 1.45pg/mL |
97% |
99.5% |
0.98 |
|
TNF-α |
> 22.31 pg/mL |
83% |
99.5% |
0.97 |
|
IL-10 |
> 11.15 pg/mL |
50% |
85% |
0.67 |
|
TNF-α/IL-10 |
> 3.55 |
77% |
93% |
0.91 |

ROC assessment of the ability of serum levels of C-reactive protein (CRP) (A), tumor necrosis factor-α (TNF-α) (B), interleukin -10 (IL-10) (C), and TNF-α/IL-10 ratio (D) to differentiate patients with premature ST-segment elevation myocardial infarction(STEMI)from healthy controls.
Changes in serum oxidative stress biomarkers in premature STEMI patients versus healthy controls
Serum T-AOC levels were significantly higher in premature STEMI patients than healthy controls (25.5 ± 0.9 versus 14.9 ± 0.4 U/mL; P > 0.001). The higher serum levels of NO (10.5 ± 0.3 versus 8.1 ± 0.3 nmol/mL; P > 0.001) and the lower GSH levels (3.8 ± 0.1 µg/mL versus 5.2 ± 0.2 µg/mL; P > 0.001) were significantly different when comparing patients and controls. Serum vitamin C levels were non-significantly higher in patients than controls (7.8 ± 0.3 versus 7.3 ± 0.3 µg/mL; P = 0.17). Serum LPO levels were markedly higher in patients than in the healthy group (15.8 ± 0.4 versus 4.1 ± 0.2 µmol/L, P > 0.001) (
The association of the inflammatory and oxidative stress biomarkers with the disease severity assessed by the SYNTAX score
There were significant progressive increases in serum levels of CRP (P > 0.001), TNF-α (P > 0.001), and NO (P = 0.04) with the progress in disease severity assessed by SS (
Receiver operating characteristic (ROC) analysis of serum levels of the biomarkers
ROC was utilized to determine the cutoff levels, sensitivity, specificity, and area under the curve (AUC) to investigate the ability of CRP, TNF-α, IL-10, and the TNF-α/IL-10 ratio to differentiate premature ST-segment elevation myocardial infarction (STEMI) patients from healthy controls. A serum CRP cut-off point >1.45 pg/mL had a sensitivity of 97% and a specificity of 99.5% for the prediction of premature STEMI, with an AUC of 0.980. A serum TNF-α cutoff point >22.31 pg/mL displayed a sensitivity of 83% and a specificity of 99.5% in predicting premature STEMI, with an AUC of 0.970. Serum IL-10 levels >11.15 pg/mL displayed a sensitivity of 50% and a specificity of 85% for the prediction of premature STEMI, with an AUC of 0.670. A serum TNF-α/IL-10 ratio cutoff point >3.55 displayed a sensitivity of 77% and a specificity of 93% for the prediction of premature STEMI, with an AUC of 0.910 (Figure 2,
Discussion
Hypertension, dyslipidemia, diabetes mellitus, and smoking are the standard modifiable cardiovascular risk factors. Although 12.9% of patients with STEMI have been demonstrated to be risk factor-free, they exhibit more aggressive disease and adverse outcomes24. Currently, atherosclerosis, as a chronic inflammatory process, incorporates inherited and environmental factors, metabolic disorders, and infections. These risk factors initiate and promote lesion development to the extent of acute thrombotic complications and serious clinical events25, 26. Since inflammation and oxidative burden are at the core of the clinical manifestations and complications of CAD, recent preventive and therapeutic trials have demonstrated the effectiveness of anti-inflammatory treatment for cardiovascular outcomes in patients with CAD. These interventions include changes in the serum CRP levels used as the primary endpoint, cytokine inhibitors and receptor antagonists, potentiating IL-10, and scavenging oxygen-free radicals27, 28, 29. Therefore, we planned this study to assess the role of CRP, TNF-α, IL-10, and oxidative stress in premature STEMI and its correlation to disease severity in Egyptian patients.
The systemic inflammatory biomarker, CRP, is extensively used as the primary endpoint and severity outcomes gold standard biomarker in the reported CAD studies28, 30. CRP binds the membrane of ischemic/hypoxic cells and activates their phagocytosis, without giving room for their recovery and regeneration31. In our patients, CRP revealed significantly higher levels in young STEMI patients than in controls and efficiently predicted premature STEMI at levels >1.45 pg/mL with 97% sensitivity, 99.5% specificity, and an AUC of 0.98. In accordance with our results, the studies of Han and Goswami . reported significantly elevated levels of CRP in CAD patients compared to healthy controls32, 33. Furthermore, our results presented an association between the severity of coronary artery lesions assessed by the SYNTAX score and the circulating levels of CRP, where the patient group with SS > 21.5 had the highest levels. Consistently, Liu suggested that hs-CRP levels can indicate the severity of CAD assessed by SS and aid in CAD risk stratification and prognosis 1. In the presence of classic risk factors, hs-CRP levels > 6 mg/L were independently associated with the risk of cardiovascular events in aged CAD patients presented with confirmed atherosclerotic occlusive disease. Additionally, a great reduction in the 2- and 5-year event-free survival was observed for patients with risk factors but lower hs-CRP levels34. Serum levels of hs-CRP in CAD patients independently predict the risk of rapid angiographic stenotic progression35.
Serum levels of TNF-α, a major pro-inflammatory cytokine, were found to be significantly and markedly elevated in our premature STEMI patients compared to healthy controls. TNF-α efficiently predicted premature STEMI at a cutoff level >22.31 pg/mL and displayed 99.5% specificity, 83% sensitivity, and an AUC of 0.970. Similarly, Kumari et al. and Goswami et al. reported significantly elevated levels of TNF-α in CAD patients compared to healthy controls32, 36. Inhibition of TNF-α signaling is one of the investigated new approaches for preventing CAD29, 34. Levels of TNF-α > 6 pg/mL were independently associated with cardiovascular event risk in aged CAD patients with confirmed atherosclerotic occlusive disease, greatly reducing 2- and 5-year event-free survival34. Our results also exhibited a significant association between the severity of coronary artery lesions assessed by the SYNTAX score and the circulating levels of TNF-α. TNF-α and CRP are major pathogenic players in STEMI patients, and their levels correlate positively with each other, but non-significantly negatively with IL-10. TNF-α levels increase with the increase in stent numbers30, 37. Patients with angiography-confirmed CAD presented significantly higher serum TNF-α levels compared to healthy controls38. Serum levels of TNF-α in CAD patients independently predict the risk of rapid angiographic stenotic progression35.
IL-10, a cytokine with anti-inflammatory properties, presented higher serum levels in our premature STEMI patients compared to the volunteers, with a cut-off point of >11.15 pg/mL, 50% sensitivity, 85% specificity, and an AUC of 0.67. Consistently, the study by Goswami exhibited higher levels of IL-10 in MI patients than controls32. In contrast, Kumari. presented lower levels of IL-10 in CAD patients than the controls36. Several previous studies on ACS patients demonstrated a significant association between elevated IL-10 levels and adverse cardiovascular events39. Furthermore, we observed a higher TNF-α/IL-10 level in premature STEMI patients than controls, which is supported by the studies of Kumari . and Goswami .32, 36. The findings of Karu indicated that the serum levels of IL-10 were significantly lower in CAD patients of both sexes compared to healthy controls40. The study by Tajfard e revealed a significant reduction in the levels of IL-10 between angiographically-confirmed CAD patients and healthy controls41. The serum levels of TNF-α and IL-10, among other cytokines, significantly positively correlated with the severity of CAD assessed by the Gensini score42. The cardioprotective effect of paeoniflorin glycoside was correlated with significant increases in IL-10 secretion by ox-LDL-treated Human coronary artery endothelial cells through inhibiting the Wnt/β-catenin pathway43. MicroRNA‐34a is implicated in atherosclerosis by increasing macrophage cholesterol, blood lipid, inflammatory cytokines, and cell adhesion molecules. Its plasma levels correlate positively with inflammatory cytokines such as TNF-α, but negatively with IL-1044.
The higher levels of antioxidant biomarkers observed in our patients compared to healthy controls are not surprising in light of previous publications with a high range of variance. Oxidative stress biomarkers have been extensively investigated in atherosclerosis and CAD, as oxidative stress and inflammation are pathogenically implicated in the disease and its risk factors. Oxidative stress is instrumental in the initiation with the deformed ox-LDL and endothelial dysfunction, progression, and complications of CAD, and may predict outcomes independently of traditional risk factors. Quenching reactive oxygen species (ROS) is among the new approaches to combating the disease45. Although a long list of oxidative stress biomarkers has been developed and used, they lack standardization and specificity for CAD, with significant limitations that hinder their integration into clinical practice45. In our patients, NO showed elevated levels in premature STEMI patients compared to healthy controls and exhibited increased levels with increasing SS, implying a correlation with disease severity. The study by Cheraghi . supports our findings, presenting increased levels of NO in CAD patients compared to healthy controls46. NO has complex involvement in myocardial infarction and CAD. It initially increases in response to ischemia and inflammation, but its bioavailability diminishes due to endothelial dysfunction and oxidative stress, leading to vascular dysfunction26. Moreover, serum levels of T-AOC and LPO were elevated, while GSH was reduced in our premature STEMI patients compared to the healthy controls. This is consistent with the findings of Cheraghi ., where levels of GSH in healthy individuals were higher than in CAD patients46. Lower GSH levels may contribute to the development or progression of CAD, as reduced antioxidant capacity may lead to increased oxidative stress and endothelial dysfunction, both of which are implicated in CAD pathogenesis26, 46. Variations in levels of the oxidative stress biomarkers used in our study compared to previous reports could be attributed to several plausible factors that are mainly dietary, as young CAD patients may be more vigilant about their diet: the prevailing Mediterranean diet, dietary supplements, and the high nitrate/nitrite contents in Egyptian foodstuffs. In middle-aged adults like our patients, the antioxidant capacities would be more vividly induced by reactive oxidative compounds47, 48. As NO was measured as nitrite, it is possible that it rather reflects peroxynitrite, a pro-oxidant marker, that would correlate with low NO bioavailability. Also, the apparent increase in T-AOC in our patients could be a reaction to the existing oxidative stress, as reflected by the increases in LPO and reduction in GSH.
Preclinical research and extensive epidemiological studies have demonstrated the key role inflammation plays in the pathophysiology of atherosclerosis. There are multiple mediators in the intricate pathophysiological relationship between inflammation and atherosclerosis. Previous studies have demonstrated that anti-inflammatory treatments can lower the risk of cardiovascular disease. However, the results of treatments specifically targeting inflammation remain debatable; further studies are needed to better understand this association in the future29.
The limitations of our case-control cross-sectional study included the single-center small sample size, without longitudinal patient follow-up and data collection. This limits its generalization and usefulness. Furthermore, as we targeted acute STEMI patients, the biomarkers' characteristics (cut-offs, sensitivity, specificity, and AUC) are neither generalized to non-acute cases nor to the general population. The longitudinal implications of our findings were difficult to assess due to the loss of follow-up for most of our patients.
Conclusions
Our findings indicate that the inflammatory markers—CRP, TNF-α, IL-10, TNF-α/IL-10 ratio, and oxidative stress—contribute to premature STEMI development in the Egyptian population, and they are correlated with the severity of coronary artery lesions. They revealed biomarker potential; strongest for CRP, followed by TNF-α, then TNF-α/IL-10 ratio, and weakest for IL-10. Future studies may target a deeper understanding of the underlying mechanisms of our observations and investigate other populations.
Abbreviations
ACS – Acute Coronary Syndrome, AUC – Area Under the Curve, ALP – Alkaline Phosphatase, ALT – Alanine Aminotransferase, AST – Aspartate Aminotransferase, BMI – Body Mass Index, BUN – Blood Urea Nitrogen, CAD – Coronary Artery Disease, CK-MB – Creatine Kinase-MB, CRP – C-reactive Protein, cTnT – Cardiac Troponin T, ECG – Electrocardiogram, GSH – Reduced Glutathione, HDL-C – High-Density Lipoprotein Cholesterol, hs-CRP – High-sensitivity C-reactive Protein, IL-10 – Interleukin-10, LDL-C – Low-Density Lipoprotein Cholesterol, LPO – Lipid Peroxidation, MDA – Malondialdehyde, MI – Myocardial Infarction, NO – Nitric Oxide, PCI – Percutaneous Coronary Intervention, ROC – Receiver Operating Characteristic, SPSS – Statistical Package for the Social Sciences, STEMI – ST-segment Elevation Myocardial Infarction, SYNTAX – Synergy Between PCI with Taxus and Cardiac Surgery, TAC – Total Anti-Oxidative Capacity, T-AOC – Total Antioxidant Capacity, TNF-α – Tumor Necrosis Factor-alpha, WSS – Wall Shear Stress
Acknowledgments
We highly appreciate the help in conducting this study and finalizing this manuscript, offered by Prof. Dr. Tarek H El-Metwally, Biochemistry Division, Department of Pathology, College of Medicine, Jouf University, Sakaka, Saudi Arabia, and, the Department of Medical Biochemistry, Faculty of Medicine, Assiut University, Assiut, Egypt, and, Prof. Dr. Hossam El-Din M Omar, Department of Zoology and Entomology, Faculty of Science, Assiut University, Assiut, Egypt, and, the Department of Basic Sciences, School of Biotechnology, Badr University at Assiut, Assiut, Egypt.
Author’s contributions
Omnia HM Omar and Marwa A Gaber designed the study protocol development, performed all lab investigations, and writing of the manuscript. Marwan S Mahmoud selected the participants, performed clinical assessments of all patients, interpretation of clinical findings, and participated in revising the manuscript. Abdel-Raheim MA Meki, Ahmed Y Nassar and Ayman KM Hassan revised the manuscript. All authors agreed to be accountable for every aspect of the study and accepted the final version of the article as submitted.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was carried out in compliance with the Helsinki Declaration principles after being approved by the Institutional Review Board, Faculty of Medicine, Assiut University (Approval#: IRB17200689). Volunteering participants signed the informed consent form,after being briefed on the nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

