Nipah Virus: Transmission Dynamics of a Zoonotic Outbreak and Therapeutic Challenges
- Medical Bionanotechnology, Faculty of Allied Health Sciences, Chettinad Hospital & Research Institute (CHRI), Chettinad Academy of Research and Education (CARE), Kelambakkam, Chennai, TN-603103, India
- Medical Bionanotechnology Lab, Department of Obstetrics and Gynaecology, Centre for Global Health Research, Saveetha Medical College, Saveetha Institute of Medical and Technical Sciences, Thandalam, Chennai, India
Abstract
Nipah virus (NiV) is a deadly zoonotic virus that has caused multiple outbreaks since it was first identified in Malaysia in 1998. It is primarily transmitted by fruit bats and can spread among humans, leading to severe neurological and respiratory complications. With fatality rates ranging from 40% to 75%, NiV poses a serious public health threat, and there are currently no approved treatments or vaccines. The virus enters human cells via ephrin-B2 and ephrin-B3 receptors, causing extensive harm while evading the immune system, which complicates treatment efforts. Environmental changes, including deforestation and increased human–wildlife contact, have heightened the likelihood of NiV outbreaks. Preventing future outbreaks necessitates early detection, strict biosecurity measures, and concerted global efforts to develop antiviral therapies and vaccines. This review comprehensively examines NiV's virology, transmission dynamics, clinical manifestations, and the latest advances in therapeutic care, encompassing One Health strategies and preventive measures.
Introduction
Nipah virus (NiV) is a single-stranded RNA virus that belongs to the Henipavirus genus of the Paramyxoviridae family. It is a deadly zoonotic virus that can cause fatal encephalitis, severe respiratory problems, or neurological disorders in humans1. Its high pathogenicity, which is the key factor in its classification as a Biosafety Level-4 (BSL-4) pathogen, underscores the severity of NiV. NiV was named after the first outbreak in 1999 in Sungai Nipah village in Negeri Sembilan, Malaysia. The first outbreak of NiV was reported in Malaysia and Singapore in 1999, with the virus transmitting from bats to pigs and then to humans2. NiV can infect humans through various routes: the urine or saliva of infected animals or bats, direct contact with infected humans, and the consumption of food contaminated by animals. The disease typically progresses from mild symptoms such as headache and sore throat to more severe ones like coughing, fever, drowsiness, vomiting, seizures, coma, and encephalitis, which can be life-threatening if not addressed early, usually within two to four weeks3.
NiV outbreaks are most commonly found in the southern regions of Asia, including Malaysia, Singapore, the Philippines, India, and Bangladesh. The Pteropus genus, also known as flying foxes, is the natural host of NiV. These fruit bats act as asymptomatic carriers, leading to recent outbreaks in Bangladesh, India, and especially in Kerala, India, due to consuming contaminated palm sap. A NiV outbreak was recorded in 2018 in the Indian subcontinent, particularly in Kerala, with 23 infected individuals4.
Notably, Kerala is geographically distinct from Malaysia, where the first outbreak occurred. A total of 23 patients were admitted to two different hospitals in the Kozhikode district, and 18 of these 23 patients tested positive for NiV via qRT-PCR; among them, 60% were male. All admitted patients presented with fever, hypertension, segmental myoclonus, and segmental sweating, followed by a quarantine period of two weeks. Of these 23 patients, 19 had primary contact with the index case (a 26-year-old male), and three secondary cases were in contact with the primary host in the hospital. The Indian Council of Medical Research (ICMR) confirmed that the primary infection host was from the Pteropus bat species through RT-PCR tests. All infected patients died in the same month of infection. Despite numerous outbreaks in various countries in southern Asia, NiV still lacks effective therapeutic strategies5.
Scientists are actively engaged in ongoing research to understand how NiV causes and spreads disease. To study its effects, researchers use animal models like golden hamsters, African green monkeys (AGMs), and mice. Advanced techniques, such as genetically modifying the virus to remove certain proteins, have helped identify key factors in its pathogenicity. Among these models, AGMs closely mimic the severe respiratory and neurological symptoms seen in human infections, showing a high mortality rate similar to real outbreaks. Despite advancements in antiviral therapies and initiatives to develop vaccines for NiV, there is a significant gap in moving from preclinical studies to larger human trials. Further research and awareness are essential to understand the virus's complex nature, immune evasion mechanisms, and wide host range, which requires additional studies and real-world evaluations. Hence, this review delineates NiV’s structure, replication cycle, cell-entry mechanisms, and unpredictable outbreaks, emphasizing the need for collaboration in combating NiV. Finally, it also discusses current diagnostic and therapeutic strategies for NiV and the experimental vaccines under development.
Discovery and Structure of NiV
Discovery of NiV
Over 60% of human infectious diseases, including emerging ones, come from animals. In recent decades, outbreaks of these diseases are becoming more frequent and pose a risk of turning into pandemics6, 7, 8, 9. For centuries, viral zoonotic diseases like bird flu, swine flu, SARS, MERS, Ebola, Zika, and Nipah have caused major global health crises10, 11, 12, 13. In 1999, a patient suffering from encephalitis was admitted to a hospital, marking the beginning of a meticulous investigative process. Virologists from the University of Malaysia, with unwavering dedication, isolated a new and rare virus from the patient’s cerebrospinal fluid5. This virus, which would later be known as the Nipah Virus (NiV), was isolated from three different encephalitis patients and used to inoculate Vero cells. Electron microscopy (EM) studies of this inoculated virus revealed that it had characteristics similar to those belonging to the Paramyxoviridae family. The first isolate of this virus strain was from a fatal human case in Kampung Sungai Nipah, in the Negeri Sembilan state of Malaysia14. Later on, in 2002, the International Committee on Taxonomy of Viruses (ICTV) classified NiV as the second virus of the genus Henipavirus of the family Paramyxoviridae. NiV was classified into this family because it has characteristics similar to those of the Hendra virus (HeV), which was identified in humans and horses during an outbreak in 1994 in Australia. Initially, researchers classified HeV into the genus Morbillivirus, naming it equine morbillivirus (EMV). Future whole-genome analyses of both HeV and NiV showed that they do not share characteristics with the Morbillivirus genus. Hence, they classified these two viruses into a newly generated genus known as Henipavirus15. New species of viruses have also been introduced into this genus, including Cedar, Mojiang, and Ghana Bat virus.
Structure of NiV
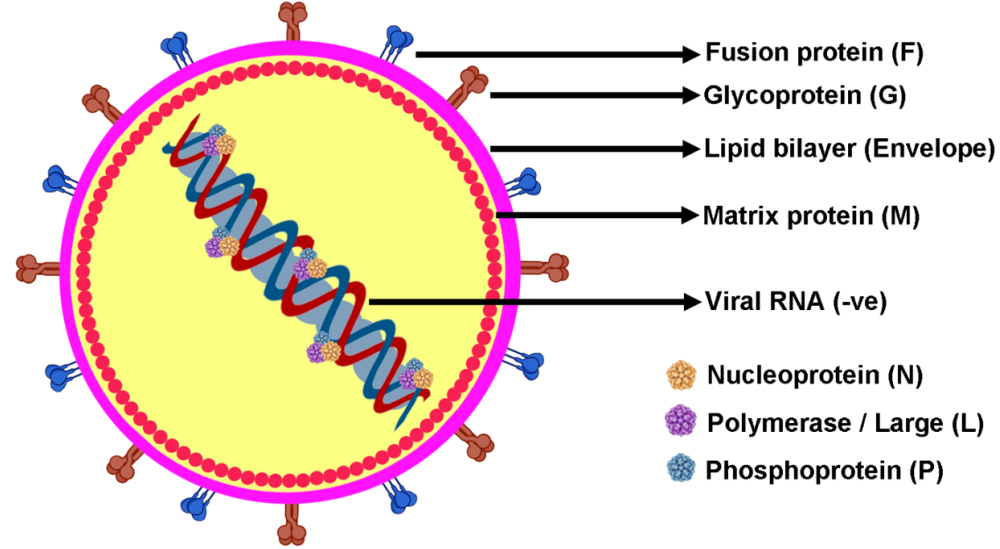
NiV has a flexible outer layer consisting of a lipid-based envelope, where two key proteins, G for attachment and F for fusion, are embedded. Inside, matrix proteins (M) form a protective shell around the viral core (Figure 1). This core holds a single strand of RNA, which is linked to nucleocapsid proteins (N) along with phosphoproteins (P), and a giant RNA-synthesizing polymerase enzyme (L)16. The virus’s genetic material consists of six main genes (M, N, P, F, G, L), each responsible for producing essential structural proteins. Through special mechanisms like post-transcriptional modifications and alternative initiation codons, the P gene can also create additional proteins (P, V, W, C). Some of these, like V and W, help the virus evade the immune system by blocking interferon production, while P, V, and W affect interferon-related signaling. The C protein mainly stays in the host cell’s cytoplasm, playing a role in the formation and release of new virus particulates16. Unlike most Paramyxoviridae viruses, NiV and HeV both do not exhibit hemagglutinin and neuraminidase activities. Instead, they rely on the interaction of their multiple proteins, like G, H, and F proteins, to attach to and enter target cells. Specifically, NiV’s glycoprotein G primarily binds to the ephrin-B2 receptor, with some interaction with ephrin-B3. These receptors are found in various tissues, including smooth muscle, the brain, lungs, placenta, and prostate. NiV remains viable for up to three days in mangoes and certain fruit juices, and for a week in date sap at 22°C. In bat urine, which serves as a natural reservoir, the virus remains viable for around 18 hours. Fortunately, it can be effectively neutralized with common disinfectants like sodium hypochlorite. While NiV can withstand temperatures of 70°C for an hour, it breaks down completely within 15 minutes at 100°C. Multiple strains of NiV have been identified from outbreaks worldwide17.

Structure of NiV to show the arrangement of genetic materials and surface compositions. The RNA-based genome forms a ribonucleoprotein complex that attaches to N (nucleoprotein), L (polymerase), and P (phosphoprotein). The genome complex is surrounded by a lipid envelope in which glycoproteins (G) and fusion proteins (F) are embedded, whereas matrix proteins (M) are found in the inner wall of the envelope.
Replication Cycle
The genomic structure of NiV RNA has consecutive arrangements of N, P, M, F, G, and L structural proteins arranged from 3′-5′. NiV’s replication cycle begins with the virion attaching to ephrin-B2 and ephrin-B3 host cell receptors via the structural G protein. This is followed by the fusion of NiV’s viral membrane with the host cell membrane, ensuring the successful incorporation of the viral genome into the host cell’s cytoplasm18. The viral RNA, along with proteins N, P, and L, forms the ribonucleoprotein complex, leading to NiV replication and transcription. The RNA polymerase enzyme transcribes the viral genome to produce mRNA for protein synthesis. The translated surface glycoproteins of NiV are inserted into the host cell’s endoplasmic reticulum for post-translational modifications. Meanwhile, other proteins (N, L, P, and M) remain in the host cell cytosol. Excess transcription of viral mRNA leads to the formation of a full-length anti-genome, producing additional copies of the NiV genome. The newly transcribed F and G proteins are incorporated into the host cell membrane, leading to the formation of new virions facilitated by M proteins18.
Cell entry mechanisms by F and G glycoproteins
F and G glycoproteins of NiV can target immune cells, endothelial cells, and neuron cells, leading to immunosuppression, vascular damage, and neurological complications19. Glycoprotein G plays a vital role in the initial stages of viral infection by recognizing and binding to specific receptors on the host cell surface. This interaction is essential for viral entry, as it facilitates the attachment of the virus to the host. The F protein, on the other hand, is responsible for mediating the fusion process between the viral and host cell membranes, allowing the viral genetic material to enter the host cell. Both glycoproteins contribute to the virus’s ability to recognize and interact with ephrin-B2 and ephrin-B3, which serve as critical receptors on host cells. Structurally, Glycoprotein G is classified as a type II membrane protein. It undergoes polymerization to form tetramers, a process driven by interactions at its N-terminal α-helical stalk domains18. Meanwhile, the C-terminal globular head of Glycoprotein G plays a key role in binding to the host cell receptor, ensuring a stable interaction necessary for viral attachment. In contrast, Glycoprotein F belongs to the class I viral fusion proteins and features a globular head composed of three distinct domains. This glycoprotein is specifically designed to facilitate membrane fusion, binding to the host cell membrane through its C-terminal α-helical stalk. The coordinated actions of these glycoproteins are crucial for successful viral entry and infection. Cui et al. studied the interaction of NiV’s structural glycoprotein F and G in HEK293T cells via proximity labeling (PL) technology20. A total of 1996 and 1524 host proteins were screened against NiV F and G glycoproteins using TurboID PL combined with LC-MS/MS to identify potential interactions in HEK293T cells. A list of 20 high-confidence NiV F and G interacting proteins were identified with three mutual interacting proteins for NiV F and G, which are Cortactin (CTTN), Serpine mRNA Binding protein 1 (SERBP1), and stathmin 1 (STMN1). Western blot results showed that all three proteins have no significant effect on cell viability, compared with control CCK8 analysis. A luciferase detection assay was performed, which revealed that overexpression of CTTN could effectively inhibit NiV infection when compared to SERBP1 and STMN1. Western blots were also performed to evaluate increasing concentrations of CTTN proteins, which proved that increasing concentrations of CTTN could effectively inhibit NiV infection (Figure 2). They also proved that the NiVpv or Nipah pseudovirus infection could be more prominent in the absence of CTTN protein via luciferase detection assay and Western blot.

Overexpressed CTTN inhibits Nipah pseudovirus-infected cells (HEK293T) in a dose-related manner. (A, B & C) Western blotting images where anti-myc primary antibodies were used along with anti-SERBP1 & anti-STMN1. The secondary antibodies were used as anti-mouse-immunoglobulin G (IgG) or HRP-conjugated anti-rabbit-IgG. (D, E & F) It shows the analysis of cell viability assay for the detection of the effects of recombinant plasmids like CTTN, SERBP1, and STMN1, along with transfection reagents. (G, H & I) Luciferase-based assay indicating CTTN overexpression inhibits NiVpv infection. (J) Western blotting images where anti-CTTN was used as the primary antibody and HRP-conjugated anti-rabbit immunoglobin was used as the secondary antibody. (K) Luciferase-based assay. Adapted from Cui
Epidemiology and Outbreaks of NiV
Epidemiology
Flying fruit bats are often considered a natural reservoir of NiV. These bats frequently feed on fruits, nectar from trees, and sap from palm trees in and around farmland, facilitating virus spillover. These bats are often found in the southern part of the Asian continent, and the disease is endemic to people in these regions as well as in the Australian continent and East African countries21. These pathogenic bats are asymptomatic and release the virus into the environment in the form of saliva, semen, urine, and excreta. Studies on fruit bats in the countries of Cambodia22, Madagascar23, Thailand24, and Ghana revealed that these bats contained NiV-neutralizing antibodies during surveillance25. NiV has been classified as a deadly zoonotic disease, and its outbreak has been recorded in all southern parts of the Asian continent since its discovery. NiV outbreaks are primarily recorded in Malaysia-Singapore, the Philippines, Bangladesh, and India (Figure 3).

Geographical location of NiV outbreak from 1999-2024 (generated from mapchart.net).
The first-ever NiV outbreak was recorded at a pig farm in Malaysia in 1998, with NiV transmission from Flying fruit bats to pigs (primary infection) and subsequent infection of workers (secondary infection) on the farm. All this began in Nipah village of Negeri Sembilan district of Malaysia in December 1998. Second and third consecutive outbreaks were reported in December 1998 and January 199926. In the outbreak between September 1998 and May 1999, 265 cases of acute encephalitis (AE) were confirmed, of which 105 died, resulting in a 39.6% mortality rate. This also resulted in the downfall of the pig-farming sector. Initially, it was diagnosed as Japanese Encephalitis Virus (JEV) due to its prevalence in these areas. People who had received the JEV vaccine showed no immunity against NiV, allowing the virus to continue spreading among the population27. A similar outbreak was observed in pigs, particularly in adult males, and many human cases were traced back to pig farms. In response, the Government implemented strict control measures, including culling infected pigs, restricting pig trade, and minimizing human contact with pigs to prevent further spread. The virus eventually reached Singapore in March 1999 when infected pigs were exported from Malaysia, leading to infections among 11 pig farmers. The outbreak then resulted in the shutting of two large pig slaughterhouses to restrict further spread of NiV. Nucleotide sequences obtained from RT-PCR tests showed similarities between samples taken from infected workers in pig slaughterhouses in Singapore and those infected in Malaysia26.
In 2014, severe illness was spreading among horses and humans in the southern part of the Philippines. Initially, two deaths of patients with unknown illnesses were reported at the National Centre of Epidemiology, Philippines (NCEP) on April 2nd, 201428. From May 22-24, 2014, an investigation was conducted with the assistance of the World Health Organisation (WHO), and individuals who were in the vicinity of the initial two patients were interviewed. The case report revealed that 17 patients were infected through close contact (11 AE, 5 influenza-like illness (ILL), 1 Meningitis). An 82% mortality rate was recorded among patients with AE, and no mortality was documented in patients with ILL and Meningitis. Similarly, from March 3rd to May 11th of 2014, 10 horse deaths were reported from the infected areas and subsequent deaths of cats and dogs were also observed. Two serum samples and seven cerebrospinal fluid samples were obtained from persons in close contact with the infected patients and tested at the Australian Animal Health Laboratory and the National Institute of Infectious Diseases (Japan) for detection of various neurotropic pathogens via neutralization assays and pseudotyped vesicular stomatitis virus possessing NiV envelope proteins. Three samples tested positive and were also subsequently confirmed by ELISA. These short-segment isolates showed a similarity of 99% to the Malaysia strain and 94-96% to the Bangladesh strain; they were further deposited in the DNA Databank of Japan (DDBJ)28.
Between 2001 and 2011, Bangladesh was hit by 11 NiV outbreaks, resulting in a total of 196 cases of NiV infection and encephalitis. The virus primarily spread through contaminated date palm sap, which was infected by fruit bats and later consumed by humans29. Following the 1998 NiV outbreak in Malaysia, Bangladesh ramped up its surveillance efforts. The Institute of Epidemiology, Disease Control and Research (IEDCR) and the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) took the lead in monitoring potential cases. Their approach included tracking reports of unexplained deaths through 10 national newspapers and 8 television channels, alongside a surveillance program in 10 government hospitals. The country’s first confirmed NiV outbreak occurred in 2003 when the CDC in Atlanta and icddr,b identified the virus in patients who had died from unknown illnesses in Meherpur (2001) and Naogaon (2003)30. Since then, 11 consecutive outbreaks of NiV have been reported in Bangladesh, with 196 cases from 20 districts and a 77% mortality rate. Reports showed that 63% of these cases were adult males with a median age of 25. A case study revealed that almost all of these patients had consumed infected date palm sap from flying fruit bats 30 days before their onset of illness. Flying fruit bats (Pteropus bat) from this area also tested positive for NiV antibodies. A zoonotic investigation team from icddr,b reported the contamination of date palm sap from the shaved side of date palm trees by infrared cameras31. A recent report from IEDCR, Bangladesh 2015 stated that nine Nipah cases were found with a 67% mortality rate from six different states, namely Nilphamari, Ponchoghor, Faridpur, Magura, Naugaon, and Rajbari, with a median age of 13 years and 56% of cases in adult males32. In the last couple of years, seven cases were reported in 2020, two in 2021, eleven in 2023, and two laboratory-confirmed cases in 2024, as reported by WHO in Bangladesh.
Siliguri, a district in West Bengal bordering India and Bangladesh, was the first region to report a devastating NiV outbreak in India. Eighteen samples from the Siliguri district were tested; nine serum samples showed positive for NiV-specific antibodies, and five urine samples showed the presence of NiV strains. This outbreak led to 66 infected individuals with a 68% mortality rate33. The most recent outbreak of NiV occurred in 2018 in India in the Kozhikode district of Kerala34. On May 17, 2018, a 28-year-old male was admitted to Kozhikode Hospital with myalgia, fever, and headache along with his father and aunt, who were in close contact with him. Three clusters of cases appeared during this period, May 4-19, 2018. Twenty-three people were infected from index cases, with a mortality rate of 91%. Eighteen of these 23 cases showed positive for NiV by RT-PCR. Serological analysis was carried out on 18 patients; nine patients had IgM and four had IgG antibodies. Five cases showed no presence of IgM or IgG antibodies. Phylogenetic analysis using Next-generation sequencing (NGS) identified the genes encoding the NiV. After three years, in 2021, the Kozhikode district of Kerala again had sporadic outbreaks of NiV infection from an index case of a 12-year-old boy35. From this index case, three different clusters of NiV cases were spread in three different hospitals involving a total of 64 contacts. Virologists also tested bat species such as Pteropus medius and Rousettus leschenaultia in the outbreak area for the presence of anti-NiV IgG antibodies. A qRT-PCR was carried out on 64 people who were in close contact with the index case. Out of 64, 59 were asymptomatic, and 5 were symptomatic, confirmed by ICMR-NIV Pune. Tests on bat species showed the presence of anti-NiV IgG antibodies: 37.73% in R. leschenaultia and 21% in P. medius. NiV neutralizing antibodies were found in P. medius. Whole-genome sequencing (WGS) from the index case revealed 95% similarity to the Nipah Ⅰ genotype sequence reported earlier in Kerala. India announced its fifth outbreak of NiV in Kerala in July 2024, after the passing of a 14-year-old boy.
Socioeconomic impact
In Bangladesh and India, monitoring for NiV is not only a public health concern but also a socioeconomic requirement. These nations frequently experience outbreaks that put additional strain on their already limited healthcare resources, particularly in rural and lower-income areas. Even though there is a chance that raw date palm sap could be contaminated with NiV, many families in Bangladesh depend on harvesting and selling it for income. This leads to a challenging trade-off between livelihood and health. Public health campaigns aim to raise awareness, but changing behavior is difficult due to ingrained cultural customs and financial reliance. States like Kerala in India have made strides in early detection and isolation through stronger healthcare systems. Even so, outbreaks can swiftly overwhelm local infrastructure and cause disruptions to daily life and work, particularly in low-income communities.
When comparing South and Southeast Asia, the situation becomes even more complex. Stronger economies and improved healthcare systems have lessened the impact of NiV in nations like Singapore and Malaysia. There, animal farming industries were frequently implicated in outbreaks, and authorities were able to take prompt action once the connection was established. Bangladesh and India, on the other hand, are burdened with a lack of resources, inadequate access to modern healthcare, and economic activities that inadvertently increase exposure, such as collecting raw sap or selling street fruit. Furthermore, the greater fatality rates in South Asia are a result of deficiencies in crisis response capabilities, medical access, and education in addition to biological variations in the virus. Socioeconomic factors, including poverty, educational attainment, and healthcare access in rural areas, significantly influence how severely communities are impacted and how quickly they can recover.
Transmission of NiV
Interspecies Transmission
() is the major natural reservoir of NiV in South Asian countries. Bangladesh experiences frequent NiV outbreaks, mainly from January to May, owing to interspecies transmission from animals to humans. The country documents the majority of global NiV cases, primarily associated with the consumption of raw date palm sap, a seasonal delicacy (Figure 4). Sap is collected overnight from shaved palm tree branches using mud pots and is sold before fermentation begins. During overnight harvesting, date palm sap becomes contaminated by P. medius bats through feces, urine, and saliva. NiV can survive up to four days in bat urine but only 24 hours in sap. Outbreaks peak in winter, as food scarcity drives bats closer to villages, increasing contamination. This season also raises viral loads in bats and weakens their immune systems, contributing to outbreaks36. Malaysia was the first South Asian country to experience a NiV outbreak through an intermediate host—infected pigs. Pigs contracted NiV from P. medius bats via spillover or contaminated fruit. Farm and slaughterhouse workers became infected through direct contact with pigs or pig meat. The outbreak spread further when infected pigs were exported to Singapore, causing a 1999 outbreak there37, 38. India experienced the most sporadic outbreaks in 2001 and 2007 in the Siliguri and Naida cities of West Bengal. The NiV strain isolated in the Indian outbreak had a higher mortality rate than all other strains isolated worldwide39.

A) Bats contaminate palm sap, which is consumed by humans, representing a major transmission route in India and Bangladesh. B) Spillover events from bats to horses affect farmers, representing a major transmission route in the Philippines. C) Bat-bitten fruit consumed by pigs is a major transmission route in Singapore and Malaysia.
Intra-species Transmission
The high fatality rate and the severe outbreak of NiV in Bangladesh led to the discovery of human-to-human transmission, which contributed to increased mortality and prevalence of infection in Bangladesh. Apart from contaminated raw date palm sap, other sources of NiV infection in Bangladesh include bat hunting and the consumption of bushmeat40. Although Bangladesh's primary infection source has not been definitively identified, it is assumed to be transmitted from bat species. In India in 2021, an outbreak of NiV began with an index case of a 26-year-old male, leading to three different clusters in three different hospitals that had contact with the index case35. In Bangladesh and India, airborne transmission is the major route of NiV infection among caretakers and doctors who come in contact with the infected, and there is also a high chance of NiV infection. In Kerala, which is more prone to human-to-human transmission, the government took the initiative to incinerate the corpses of infected individuals or bury them at a depth of 10 feet using personal protective equipment (PPE) to minimize the spread25.
Pathogenesis of NiV
The pathogenesis of NiV is not fully understood worldwide, largely due to the lack of diagnostic systems, the rapid evolution of the disease, and the absence of a high-level research containment facility (BSL-4) for its study. The pathogenesis of NiV is a complex, multi-stage process, contributing to its severity. NiV infection begins in the human body when its structural proteins, NiV G and F, bind to the ephrin-B2/B3 receptors on respiratory epithelial cells. After overcoming the initial defense of endothelial cells, it establishes a foothold in alveolar and bronchiolar tissues, initiating an immune response41. The NiV outbreak in Kerala (NiV-B) resulted in events resembling acute respiratory distress syndrome (ARDS). This immune response released an array of cytokines, chemokines, and signaling molecules to combat NiV infection. Infected patients experienced respiratory discomfort, compromised lung function, and various respiratory symptoms due to the immune response to NiV. As the infection spreads, the airway’s epithelial cells release compounds such as granulocyte colony-stimulating factor and interleukins to combat it. These mediators further worsen the infection’s pathological condition and aggravate the patient’s respiratory symptoms42. NiV extends from the respiratory system to the bloodstream and various organs by interacting with the endothelial cells lining the blood vessels, potentially resulting in multi-organ failure. NiV infection in humans also exhibits neurotropism by directly attacking neural tissues. Additionally, it infects the Central Nervous System (CNS) through neurotrophic factors and blood vessels. Entry via blood vessels can profoundly impact the blood-brain barrier (BBB), which acts as a primary defense against pathogens infecting the brain43. Researchers have also identified an alternative route of NiV infection into the CNS via the olfactory nerve. Once NiV reaches the olfactory nerve, it can disseminate throughout the ventral cortex of the brain, exacerbating neurological symptoms and posing a significant threat to the infected individual44, 45.
Clinical Manifestations of NiV
NiV outbreaks vary in incubation periods across regions. In Malaysia, the incubation period ranged from 2 days to 4 months, while in Bangladesh, it was typically under 10 days. Infected individuals can be asymptomatic or experience severe symptoms including fever, mood changes, fatigue, dry cough, respiratory distress, convulsions, and vomiting. During the 1998 Malaysian outbreak (NiV-M), approximately 8% of cases were asymptomatic, as confirmed by laboratory studies. In contrast, infections in Bangladesh often lead to mild to severe symptoms, particularly respiratory issues and encephalitis. Although NiV-M and NiV-B are distinct strains, they share about 91.8% similarity at the genetic level. NiV-B, however, tends to be more virulent, with fatality rates between 40% and 75%. It may also spread more easily between people compared to NiV-M. The two strains differ in their modes of transmission, incubation periods, mortality rates, and clinical presentations. The NiV-B strain, which spreads more readily from person to person, caused more respiratory problems than NiV-M. India, in particular, recorded a markedly high number of respiratory cases linked to NiV-B, underscoring the severity of the outbreak46. NiV-B infection affects the respiratory system and can cause severe nervous system damage and encephalitis. MRI is crucial for assessing brain involvement. In Malaysia, NiV-M-infected patients exhibited extensive lesions in the pons, cortex, lobes, and temporal regions, leading to inflammation and cortical damage. In Singapore, MRI scans revealed smaller, approximately 1 cm lesions bilaterally, primarily in deep white matter and subcortical regions47.
Diagnosis of NiV
Diagnosing the disease during an epidemic is crucial for controlling the outbreak among the general population, limiting its spread, and providing the necessary care for patients. NiV outbreaks can be diagnosed via many direct detection methods, including virus isolation, nucleic acid amplification, immunochemistry or immunofluorescence assays, and sequencing. Indirect detection methods include enzyme-linked immunosorbent assay (ELISA), anti-NiV IgM or IgG antibody detection, and virus-neutralization tests. However, qRT-PCR (quantitative reverse-transcription polymerase chain reaction) is widely utilized worldwide for the initial diagnosis of NiV within a few hours of sample collection48. qRT-PCR is currently the most reliable method for detecting acute NiV infections because it is highly specific and sensitive. Samples such as nasal swabs, urine, cerebrospinal fluid, and blood are commonly used for testing. Most molecular tests target the N gene of the virus39. However, the sensitivity of the test can vary depending on the platform; RT-PCR can detect around 10³ viral copies per reaction, SYBR Green–based assays are more sensitive at around 20 copies, and multi-pathogen card arrays can detect as low as 54 copies per reaction. Because there is no international standard for NiV testing yet, comparing the accuracy of different methods remains a challenge.
Virus Isolation (VI)
VI was the initial diagnostic method prior to qRT-PCR for the confirmation of NiV infection. VI requires a BSL4 cabinet for isolation and characterization, as it is a highly virulent and pathogenic strain. The isolation process is time-consuming and laborious and exhibits lower sensitivity compared to qRT-PCR, because there is a reduced probability of isolating a virus strain from the sample. Hence, it is widely used as a confirmatory diagnostic method, while qRT-PCR serves as the primary diagnostic method. Strains isolated by this method are mostly used to understand their underlying infection mechanism and vaccine development49. Samples from throat and nasal swabs, urine, and cells cultured from lung and brain tissues are amplified using Vero E6 cell lines as a host. After 3 days, a cytopathic effect appears, resulting in the subsequent formation of syncytia cell lines. These cells detach from the adhesive surface, leaving a hole. This technique is used to differentiate between NiV and HeV because the nuclei and nucleocapsid of NiV are attached peripherally, whereas those of HeV are attached centrally50.
Types of diagnostic methods of NiV
|
Detection methods |
Types |
Outcome |
Ref. |
|---|---|---|---|
|
Initial detection method |
qRT-PCR |
They developed a quadruple fluorescence-based qRT-PCR for the detection of viruses such as the Langya virus, Mojiang virus, Nipah, and Cedar virus from the Henipa family. They developed specific primers and tagged it fluorescence tags for its detection. The optimal linear detection range for all these viruses is between 101 -108 copies/µl, and the lower detection limit was 10 copies/ µl. This method exhibits good sensitivity, selectivity, and repeatability. |
|
|
Direct detection method |
Immunofluorescence assays |
Henipavirus-B2 receptor was used as the capture ligand and with two different monoclonal antibodies (mAbs), namely F27NiV-34, which detects NiV and HeV, where F20NiV-65 was used to detect NiV. Later on, using this assay, a lateral flow strip was developed using ephrin B2 as the capture ligand and F20NiV-65 as the capture ligand for NiV detection. |
|
|
Direct detection method |
Sanger sequencing |
The reverse transcription loop-mediated isothermal amplification (RT-LAMP) method was developed to detect NiV rapidly. A pseudo virus of NiV was utilized to replicate live NiV. RT-LAMP primers were designed to target the conserved gene of the N viral genome of NiV. This assay showed a detection limit of 100pg total pseudovirus 10 folds higher than the conventional RT-PCR. |
|
|
Direct detection method |
ELISA |
Two antigen-based ELISA (AGLISA) were developed with the help of the Ephrin-B2 receptor of Henipavirus and monoclonal antibodies (mAbs) to detect NiV and HeV. This method showed a diagnostic percentage of 100% for NiV and 97% for HeV. Samples from 4 days post-infection of pig nasal cavities were tested, and this method could effectively discriminate between NiV and HeV. |
|
|
In-direct detection method |
NiV IgM or IgG antibody detection |
An ELISA method for NiV IgM or IgG was developed to detect specific antibodies in human sera samples. Results showed 99.28% and 100% percentage similarity for IgG and IgM antibodies with reference tests from CDCP, USA. |
|
|
In-direct detection method |
Virus-neutralization tests |
They developed a neutralization assay method with the help of Vesicular stomatitis virus and fluorescent protein. This assay helps in detecting the presence of NiV-neutralizing antibodies for both the NiV-M strain in the hamster sample and the NiV-B strain in human serum samples. Hence, this assay could be useful for detecting neutralizing antibodies for other paramyxoviruses. |
|
Advancements in Therapeutic Strategies
Currently, there are no approved vaccines or treatments for Nipah virus infection worldwide. Existing therapeutic options are limited to supportive hospital care and experimental interventions, including antiviral drugs and monoclonal antibodies.
Antiviral drugs
Several antiviral drugs have been explored and studied for their efficacy against NiV infection. Remdesivir is an antiviral prodrug with a wide range of RNA polymerase inhibition capabilities. Lo . studied the efficacy of Remdesivir against NiV-B strain in AGMs57. NiV was isolated from a patient in Bangladesh and used to infect animals intranasally and intratracheally with Tissue Culture Infectious Dose (TCID50). Animals were grouped into treatment and control groups, each consisting of two male and two female monkeys, and treated with Remdesivir in a vehicle solution (12% sulfobutylether-b-cyclodextrin in water and hydrochloric acid (pH 3.5)), while the control group received vehicle solution alone post 24 hours of inoculation and continued for 12 days. Only two animals from the treatment group exhibited mild respiratory disease, whereas animals from the control group had lethal respiratory issues, demonstrating that Remdesivir is a promising antiviral treatment for NiV infection. Wit et al. also demonstrated that administering Remdesivir to infected monkeys after 3 days post-infection significantly improved their NiV challenge outcomes58.
Favipiravir (T-705) is an antiviral purine analog approved in Japan to treat influenza. Favipiravir has shown its therapeutic efficacy against a wide range of RNA viruses from the families of Arenaviridae, Filoviridae, Paramyxoviridae, and the order Bunyavirales59. Dawes conducted a study in a Syrian hamster model to test the efficacy of Favipiravir against NiV infection60. Animals were inoculated with NiV-M strain and treated with Favipiravir for 14 days. They did not develop any clinical signs throughout the experimental duration or show any pathological changes, demonstrating that Favipiravir efficiently inhibits the replication of the NiV-M strain. A broad-spectrum nucleoside analog was widely used in the 1998 Malaysia-Singapore outbreak. Ribavirin is commonly licensed for treating hepatitis C, hemorrhagic fevers, and respiratory syncytial virus infection, and it is also listed on the WHO Model List of Essential Medicines61. From September 1998 to June 1999, Chong and colleagues treated 265 patients with ribavirin, while 54 patients (who refused ribavirin or did not have access to it) served as controls62. Results showed that both groups had the same level of disease development, but the mortality rate was much reduced in the ribavirin-treated group. This broad-spectrum antiviral, as listed by the WHO, is the only drug used clinically to treat Nipah virus (NiV) infections. It showed a 36% mortality reduction in the 1998–1999 Malaysian outbreak but produced mixed results in subsequent studies. Initially, ribavirin was only given orally, with the intravenous (IV) option introduced later. In the first trial, ribavirin seemed promising, and it was linked to a drop in deaths. However, a later study showed no real improvement in survival, even though most patients received it. In lab animals like hamsters, ribavirin did not lower death rates when used with chloroquine, even though both drugs worked well in vitro49, 63. During the 2018 outbreak in Kerala, India, only two of six patients treated with ribavirin survived, while all six patients who did not receive the drug died. Still, the group was too small to draw definitive conclusions about ribavirin's effectiveness64 . Some healthcare workers also took it after being exposed and did not get sick, though most experienced side effects. Unfortunately, when tested in animals such as hamsters, ribavirin did not show significant benefits, whether alone or in combination with chloroquine64. Overall, while it has been used, we still lack definitive evidence of ribavirin’s true efficacy against NiV.
Monoclonal Antibodies (mAb)
The m102.4 antibody is the most promising form of mAb therapeutics against NiV infection. The m102.4 works by inhibiting the attachment of surface glycoprotein G to the epithelial cells via Ephrin-B2 and B3. The first study using m102.4 against NiV infection was conducted by Bossart et al. in a ferret animal model65. Animals were classified into two groups (control and treatment) and exposed to a NiV dose of 500 TCID50 via intranasal and oral administration to mimic the natural route of infection. Animals in the treatment group received m102.4 24 hours prior to and 10 hours post NiV infection. All ferrets in the control group died, whereas those in the treatment group were protected from infection. Since then, mAb m102.4 has been utilized as a post-exposure treatment in India, Malaysia, Singapore, Australia, the USA, and Bangladesh16.
A humanized mAb can bind to the F glycoprotein in the NiV structure to prevent the virus-host cell membrane interaction and inhibit viral penetration into the host cell membrane by blocking key F glycoprotein epitopes66. Mire isolated a potent mAb (h5b3.1) from humans to inhibit NiV and HeV spread in a post-viral setting in ferret models67. NiV-infected ferrets were treated with antibodies by the I.P. route on days 1 and 3, and an additional group received antibodies on days 3 and 5. All treated ferrets showed weight gain, no signs of NiV infection, and no observable changes during necropsy in histopathology studies. In contrast, ferrets in the control group were euthanized due to severe clinical signs such as loss of appetite, facial edema, head and neck myoclonus, and nasal and ocular discharge. Clinical pathology analysis revealed hypoalbuminemia, lymphopenia, and thrombocytopenia in the control group. Plaque assays confirmed the presence of viral load in blood and in tissue samples isolated from the control group on or after day 8 post-infection, whereas no virus load was found in the treatment groups.
Peptide Fusion Inhibitors
Cholesterol-tagged peptide fusion inhibitors: this therapeutic approach for NiV uses a cholesterol tag in the backbone to inhibit the cell entry mechanism of NiV's F glycoprotein. This leads to structural changes in the host cell by blocking virus-mediated host cell penetration68. Porotto proved that adding cholesterol (chol) tags to HRC (C-terminal heptad repeat regions) peptides (VIKI-PEG4-chol) could effectively inhibit the fusion of NiV's F glycoprotein into the host cell membrane69. NiV strains were inoculated into Golden Syrian hamster models intraperitoneally; animals exhibited 100% resistance against NiV infection when treated with VIKI-PEG4-chol peptides two days before infection. efficacy of VIKI-PEG4-chol was tested in both Syrian hamster models (S.H.M) and cotton rats (C.R.), demonstrating that peptide half-life was longer in S.H.M than in C.R. Moreover, a peak concentration of 120 nM and 170 nM was observed in the lung and endothelium 8 hours after injection, gradually decreasing to 40 nM and 130 nM at 24 hours. The peptide was first detected in the brain at 8 hours (34 nM), increasing to 190 nM at 24 hours.
Inhalable peptide fusion inhibitors: They offer advantages by targeting the main route of NiV exposure. Mathieu . demonstrated this in both AGMs and Syrian Golden hamster models, noting a 33% higher mortality rate in Syrian Golden hamster models than in AGMs70.
Vaccines
The NiV outbreak represents a significant health threat to Southeast Asian countries due to the lack of NiV vaccines. Hence, developing vaccines against deadly pathogens involves high biosafety risks, including the handling of live pathogens and various routes of administration. Therefore, researchers have focused on one promising approach: using subunit vaccines that contain fragments isolated from NiV proteins, such as glycoprotein (F and G), tested in animal models for their therapeutic efficacy71 (
Studies on NiV vaccines that contain subunits of NiV
|
Vaccine |
Study model |
Route of Administration |
Study duration |
Outcomes |
Ref |
|---|---|---|---|---|---|
|
Adenovirus-based vaccine (AdC68-G) |
BALB/C mice |
Intramuscular/ intranasal |
68 weeks |
AdC68-G and DNA-G vaccines elicited effective cellular and long-term humoral response immune responses in BALB/C mice. |
|
|
Adenovirus-based vaccine (AdC68-G) and DNA vaccine (DNA-G) |
Syrian Golden Hamsters |
Intramuscular/ electroporation |
3 weeks |
In Syrian Golden hamster models, heterologous immunization DNA-G/AdC68-G vaccine resulted in higher T-cell responses and neutralizing antibody titers. |
|
|
HeV-sG Vaccine |
AGMs |
Intratracheal, Intranasal |
84 days |
This study reveals that 0.1 mg of the HeV-sG vaccine, formulated for humans, showed effective and protective responses in AGMs. |
|
|
Nipah virus -Virus-like particle vaccine (NiV-VLP) |
Syrian Golden Hamsters |
Intraperitoneal |
58 days |
This study used two approaches, one in a three-dose schedule and one in a single-dose schedule. Animals in the three-dose schedule (days 0,21,42) inoculated with 16000 pfu (plaque forming units) showed no changes in temperature or body weight and also all animals survived. In the single-dose schedule, IgM titers on day 14 and day 28 were gradually reduced in the vaccinated group animals. |
|
|
ChAdOx1- vectored vaccine |
Syrian Golden Hamsters |
Intraperitoneal |
70 days |
ChAdOx1 vaccine was tested against both NiV Bangladesh and NiV Malaysia strains. Prime and prime/boost regime vaccination studies proved the vaccines' efficacy against NiV strains and partial efficacy against the Hendra virus. |
|
|
NIPARAB (Rabies virus / Nipah virus) Vaccine |
mice |
Intranasal |
40 days |
The NIPARAB vaccine showed increased humoral immunity in mice with high titers of antibodies against NiV G. |
|
|
PHV02 Chimeric vaccine |
ICR mice, Syrian Golden Hamsters |
Intracardiac |
8 weeks |
The PHV02 vaccine showed minimum toxicity to NiV-infected adult mice and hamsters, making it a potential candidate for the NiV G vaccine in humans. |
|
|
LC16m8 |
Syrian Golden Hamsters |
Intramuscular |
4-6 weeks |
LC16m8 vaccinia virus expresses neutralizing antibodies against NiV, and the antibody concentration was higher than that of any other poxvirus vaccine. |
|
|
Vesicular stomatitis virus-Nipah virus (VSV-NiV G, VSV-NiV F) |
AGMs |
Intranasal, Intratracheal |
35 days |
VSV-NiV F vaccine humoral response along with complete homologous protection and partial heterologous protection. Both vaccines showed reduced NiV population in main organs such as the lungs, central nervous system, and Upper respiratory tract. |
|
Some vaccines are also being evaluated in clinical trials (Table 3). Currently, no vaccine is approved to prevent NiV. A promising vaccine candidate, based on the Hendra virus’s G glycoprotein (HeV-sG-V), was recently tested in a Phase I trial81. Since Nipah outbreaks are rare and involve few cases, traditional vaccine approval methods may not be feasible, making it crucial to identify clear markers that indicate a vaccine is protective. In a study, Leyva-Grado . tested the vaccine in AGMs and found that certain levels of both binding and neutralizing antibodies predicted that animals and humans would survive a Nipah infection81. This study suggests a reliable immune marker, or correlate of protection, for both the Bangladesh and Malaysia strains of the virus. Moderna, in collaboration with the Vaccine Research Center at the NIAID, developed the mRNA-1215 vaccine to protect against the NiV-M strain82. It is an mRNA-based vaccine delivered in lipid nanoparticles and designed to stimulate the immune system by teaching it to recognize two key viral proteins: fusion (F) and attachment (G). A Phase I clinical trial (NCT05398796) in the United States concluded in September 2024, evaluating the vaccine's safety, tolerability, and immune response. Earlier, studies in mice showed that the vaccine induced protective antibodies not only against NiV-M but also NiV-B, and even provided some cross-protection against the related Hendra virus82, 83. Several studies also highlight alternative therapies, including photodynamic therapy and other formulations to tackle these disease conditions84, 85, 86, 87.
Vaccines under clinical trials for NiV (Available from: https://clinicaltrials.gov/)
|
Trial ID |
Vaccine type |
Study type |
Duration |
Phase of clinical trial |
Study population |
|---|---|---|---|---|---|
|
NCT04199169 |
Hendra virus soluble glycoprotein vaccine (HeV-Sg-V) |
Interventional |
2020-02-18 to 2022-05-06 |
Phase 1 |
192 |
|
NCT06221813 |
Recombinant vesicular stomatitis virus vaccine (PHV02) |
Interventional |
2024-01-26 to 2024-12-26 |
Phase 1 |
120 |
|
NCT05398796 |
mRNA Vaccine (mRNA-1215) |
Interventional |
2022-07-11 to 2024-10-01 |
Phase 1 |
50 |
|
NCT05178901 |
rVSV |
Interventional |
2022-01-10 to 2023-05-30 |
Phase 1 |
60 |
Immunity
The immune system is the first line of defense against any invading bacteria, viruses, or pathogens. Several cell types are involved in innate immune responses, such as monocytes, dendritic cells, natural killer (NK) cells, eosinophils, basophils, innate lymphoid effector cells, and mast cells. NiV has developed strategic ways to dodge the body’s immune response, especially the interferon (IFN-I) system that helps fight off viruses. It accomplishes this using a mix of its own proteins, such as V, P, W, and M, that interfere with key immune signaling pathways. For instance, the V protein blocks important immune messengers (STAT1 and STAT2) from triggering the antiviral response. It also interferes with MDA5, a sensor that usually initiates interferon production, to prevent the body from launching a defense. Studies using modified viruses that carry NiV proteins showed that these proteins could significantly reduce the usual immune reaction, especially IFN production and cytokine release. Another NiV protein, M, helps suppress the immune system by degrading a protein (TRIM6) that is vital for activating IFN signals. Overall, NiV uses a coordinated and complex strategy to slip past the immune system and keep spreading88. The study by Dhondt . found that intraperitoneal NiV infection in wild-type mice could potentially delete the type-I Interferon receptor (IFNAR-KO)—which confers resistance to NiV—resulting in fatal encephalitis accompanied by pathological and immunological changes89. The NiV V protein interacts with STAT1 and STAT2, among other vital proteins, leading to the downregulation of the IFN response by the innate immune system. Furthermore, the V protein also targets MDA5 (melanoma differentiation-associated protein-5), an antiviral activator responsible for initiating the IFN response against NiV, and downregulates this response by dephosphorylating MDA518. The W protein also inhibits the IFN response by blocking TLR3 (Toll-like receptor) and RLR (RIG-like receptors) signaling pathways, destabilizing IRF3 by inhibiting both TBK1 and IKK. According to research by Yamaguchi ., the C protein of NiV can effectively inhibit the IFN response by binding to IKK and impeding the phosphorylation of IRF7 while blocking TLR7/990. NiV's structural protein M can suppress the IFN response necessary for virus assembly and budding. Sometimes, excess production of cytokines at the early phase of NiV infection may result in vasculitis and encephalitis in the host91. Neutrophils are among the first immune cells that fight the viral infection, along with several other defense mechanisms, including antimicrobial peptides, reactive oxygen species (ROS) production, and neutrophil extracellular traps (NETs). NETs comprise antimicrobial peptides, proteolytic enzymes, and mitochondrial or cytoplasmic DNA. Myeloperoxidase, cathelicidin, and α-defensins are among the antimicrobial peptides in NETs. Myeloperoxidase has strong antiviral properties against NiV, whereas α-defensins have virucidal activity against both enveloped and unenveloped viruses92. Entry of NiV into the CNS (central nervous system) disrupts the blood-brain barrier (BBB) and triggers the expression of IL-1β (interleukin-1β) and TNFα (tumor necrosis factor alpha), which can lead to neurological symptoms in the host93. NiV primarily enters the host organisms through the respiratory epithelium to the pulmonary endothelium via hematogenous dissemination, either freely or by attaching to white blood cells. Along with the lungs, NiV also infects other organs, including the spleen, kidneys, and brain, resulting in multiple organ dysfunction syndrome (MODS)94. The intricate balance between the host's immune defense mechanisms and the virus's ability to evade or suppress these responses is crucial for facilitating efficient NiV replication while avoiding premature host death. Likewise, neutrophil infiltration-induced inflammation is beneficial in controlling viral replication. However, excessive neutrophil activation, leading to hyperinflammation, can cause detrimental tissue damage. Therefore, a regulated equilibrium between the protective and potentially harmful effects of neutrophil-driven inflammation is critical for the host's effective control of NiV infection95.
Challenges in Medical Research on NiV
Developing medical treatments for NiV is not particularly profitable, as outbreaks are rare and primarily affect underprivileged regions in South Asian countries. However, raising awareness among policymakers and global health leaders is crucial, as the virus poses a potential worldwide threat. To better understand and prepare for future outbreaks, researchers need to improve disease tracking in high-risk areas and collect data on how the virus spreads among bats, pigs, horses, and humans. Machine learning can also help predict which bat species may carry NiV or similar viruses, enhancing early warning systems96. A coordinated approach involving human and animal health experts, as well as wildlife officials, is key to addressing this issue. Additionally, since countries have varying regulations for vaccine and treatment approval, working closely with international health authorities will be essential to streamline the regulatory process for potential medical solutions.
Developing effective treatments and vaccines for NiV hinges on having reliable animal models that replicate human disease conditions. A recent review highlights various models used in research, each with its strengths and limitations. Currently, the three most accurate models are Syrian golden hamsters, domesticated ferrets, and African green monkeys (AGMs). Hamsters are widely used because they exhibit symptoms similar to human infection and are cost-effective to maintain. Ferrets are also commonly used, as they develop respiratory and neurological symptoms comparable to those in human cases; however, research with ferrets is complicated by inconsistent virus strains and a lack of immune response assessment tools. AGMs are considered the most relevant for testing potential treatments and vaccines because they offer valuable insights into disease progression and immune responses. They can be infected through multiple exposure routes, and an extensive array of immunological tools is available for studying their reactions. However, their use is limited by high costs, significant space requirements for BSL-4 containment, and ethical concerns. To enhance research, scientists must refine these models by standardizing virus doses and challenge strains, identifying suitable models for chronic or relapsing infections, and ensuring consistency in testing methods. These efforts will be crucial in accelerating the development of NiV treatments and vaccines.
Non-animal models like organoids and microphysiological systems offer a promising alternative for investigating Nipah virus, especially where animal models are impractical or ethically contentious. Previous studies on viruses like SARS and Zika have used these models to uncover disease mechanisms, immune responses, and possible treatments. They are cost-effective and permit co-infection experiments, but they remain limited to certain tissues and may not entirely replicate human infections. Advancing these models requires standardized methods, reagents, and virus strains.
One big challenge in understanding how viruses cause disease and in creating effective treatments is the lack of animal models that truly mimic what happens in humans. Unlike the flat, 2D cultures traditionally used in laboratories, 3D models resemble real human tissue more closely, capturing key features like cell structure and organization. Human airway organoids, derived from stem cells, are especially promising because they closely match the actual makeup and function of the human respiratory system. Unlike standard cell lines, these organoids provide a physiologically relevant setting to study how viruses interact with human cells, yielding discoveries that are more relevant to human health. Further research is needed to understand Nipah virus strain differences, immune evasion mechanisms, and long-term effects, which could accelerate treatment and vaccine development.
Strategies Followed by the Countries to Control the Outbreak
The outbreak of NiV in the early 21 century poses a significant threat to Southeast Asian countries. Hence, the WHO has provided significant guidance in these countries to help control the epidemic. Quarantine protocols during the NiV epidemic served as a crucial approach to managing the critical situation. The absence of preventive measures and the lack of people's knowledge about this virus have worsened the situation. Various preventive measures have been implemented by the countries , including avoiding the consumption of raw date palm sap, ensuring fruits are cleaned before consumption, increasing handwashing frequency, and preventing contact with infected pigs and bats97. Healthcare professionals have also been required to exercise extreme caution when handling and transporting NiV patients. Direct contact, aerosols, droplets, and airborne precautions must be strictly monitored in hospital areas. To minimize the spread of NiV within hospitals, separate quarantine areas have been provided for NiV patients to avoid transmission to other patients. Governments also trained healthcare workers on proper transport and burial procedures for deceased NiV patients. The rapid spread of NiV led to the home quarantine of numerous patients across various cities, primarily due to the lack of dedicated NiV quarantine zones in hospitals. Medical camps also occur across home-quarantined areas to enhance people's knowledge of NiV and preparedness, while providing diagnostic kits and supplies to infected individuals and alleviating panic in society98. Implementing continuous surveillance, contact tracing, community awareness programs, and focusing on animal health are possible solutions to prevent outbreaks.
One Health approach
Numerous children and adults continue to die from unidentified diseases that arise in places where people, animals, and the environment interact, making a One Health approach urgently needed worldwide. This need is further heightened by the expanding human population and rapid environmental changes. In order to address shifting patterns of illness, healthcare behaviors, and service utilization, One Health advocates for a wide-ranging, collaborative effort involving individuals, organizations, and cultures21. The One Health framework can enhance human and animal well-being by encouraging creativity, research, and collaboration. We must explore and implement the latest advancements in diagnosis and treatment to effectively protect this shared health.
Since animals are the source of about 75% of newly discovered infectious diseases, the One Health approach is particularly important for controlling outbreaks. NiV is a prime example. NiV was first discovered in Malaysia in 1998 and has since caused severe outbreaks with high fatality rates, which have resulted in the culling of over a million animals99. Since 2001, NiV has resurfaced in Bangladesh nearly every year, as we discussed, with an average mortality rate of 74%. Its symptoms range from vomiting and fever to fatal inflammation of the brain. As deforestation and urbanization increase contact, flying foxes—natural but asymptomatic carriers of NiV—pose an increasing risk of spreading the virus to humans. Contaminated fruit may be the means of transmission, leading to local outbreaks and human-to-human spread. In order to prevent future outbreaks, early detection, public education, and international cooperation through a One Health approach that integrates human, animal, and environmental health are crucial. Additionally, the development of an effective NiV vaccine necessitates considering ecological and social factors, such as environmental degradation and human-wildlife interactions.
The One Health Approach places a strong emphasis on a number of crucial tactics to successfully stop NiV outbreaks. First and foremost, it is important to maintain the health of animals. This can be done by limiting the movement of infected animals, regularly checking the health of domestic animals, and disinfecting farms to reduce the virus's spread. To guarantee a prompt response to possible outbreaks, it is also crucial to strengthen public health systems by putting in place standard operating procedures, a strong surveillance system, contact tracing, and continual training for medical personnel100. It is essential to increase public awareness through information campaigns that highlight safe food practices, contact-droplet prevention measures, and NiV transmission. Reducing human interference with wildlife habitats through current legislation can lessen future outbreaks, while environmental maintenance—such as encouraging afforestation and green zones—helps preserve climate stability and biodiversity. Last but not least, research is essential to combating new illnesses. Although a vaccine against NiV is not yet complete, clinical trials are underway to investigate possible remedies, including those initiated by the National Institute of Allergy and Infectious Diseases (NIAID).
There are a number of issues with the One Health approach to stopping NiV outbreaks that require careful consideration. Promoting cooperation between various specialists, such as ecologists, veterinarians, and medical professionals—who frequently operate independently due to different methods—is one of the main challenges. Joint education initiatives and consistent communication are required to address this. The virus's diverse symptoms present another hurdle, making early detection in both humans and animals difficult. This could be aided by sophisticated surveillance technologies and standardized reporting systems. Combining data from various sources also presents difficulties, necessitating easily accessible databases and transparent data-sharing guidelines. Furthermore, it is crucial to engage communities and raise awareness; this can be enhanced by enlisting local leaders and providing culturally sensitive instruction. Funding and resource distribution are limiting factors; therefore, efforts to enhance funding and manage resources efficiently are necessary. Human and animal vaccines are under development, but if vaccines are not yet available, antiviral medications must be on hand. Understanding the diversity of the virus through genomic surveillance can help develop more targeted preventive measures. Finally, strengthening healthcare systems in affected areas ensures prompt diagnosis and treatment, while thorough environmental studies are crucial to understanding how factors like wildlife behavior and climate change contribute to the virus's spread.
Conclusion and Future Perspectives
The Nipah virus remains a serious public health threat due to its high fatality rate, ability to spread from animals to humans, and potential to cause outbreaks. While researchers have made progress in understanding how the virus spreads and affects the body, diagnosing and treating the virus early is still challenging. Currently, there is no specific treatment beyond supportive care, although antiviral drugs and vaccines are being developed. Given the unpredictable yet deadly nature of NiV outbreaks, there is a pressing need for better surveillance, faster diagnostics, and effective containment measures.
Future research on the Nipah virus should prioritize the development of targeted antiviral treatments and effective vaccines to reduce its high mortality rate and prevent future outbreaks. Advances in molecular biology techniques, such as CRISPR-based diagnostic tools and next-generation sequencing, can significantly enhance early detection, allowing for quicker intervention and containment efforts. A One Health approach that integrates human, animal, and environmental health will be essential in identifying NiV reservoirs, monitoring virus transmission, and preventing spillover events. Since NiV is primarily transmitted from bats to humans, understanding its ecological dynamics can help mitigate future outbreaks. Surveillance programs that track viral mutations and spread patterns in both animal and human populations will be key for early warning systems. Additionally, strengthening healthcare infrastructure in high-risk regions can improve outbreak preparedness by ensuring access to rapid diagnostics, isolation protocols, and supportive care. International collaboration among researchers, public health officials, and policymakers can facilitate data and resource sharing to develop global containment strategies. Public awareness campaigns are also necessary to educate communities in affected regions about transmission risks, such as direct contact with infected animals or consuming contaminated food. Encouraging safe agricultural and livestock-handling practices can reduce human exposure to the virus. Promising and innovative treatments, such as monoclonal antibodies and RNA-based therapies, show potential for tackling NiV by integrating expertise from virology, immunology, and nanotechnology. However, these cutting-edge solutions can be expensive, limiting their accessibility to the general public. As science advances and public health efforts grow stronger, we will be in a better position to manage NiV-related risks and hopefully prevent future outbreaks. While there is not yet a dedicated guideline or global funding program specifically for NiV treatments, organizations like CEPI and the WHO are stepping up to support related research. Still, more global collaboration and funding are needed to strengthen research efforts and public health readiness, ensuring a swift and effective response to any future threats posed by this virus.
Abbreviations
AE: Acute Encephalitis; AGMs: African Green Monkeys; ARDS: Acute Respiratory Distress Syndrome; BBB: Blood-Brain Barrier; BSL-4: Biosafety Level-4; CCK8: Cell Counting Kit-8; CDC: Centers for Disease Control and Prevention; CEPI: Coalition for Epidemic Preparedness Innovations; ChAdOx1: Chimpanzee Adenovirus Oxford 1; CRISPR: Clustered Regularly Interspaced Short Palindromic Repeats; CTTN: Cortactin; DDBJ: DNA Databank of Japan; ELISA: Enzyme-Linked Immunosorbent Assay; EM: Electron Microscopy; F: Fusion Protein (NiV structural protein); G: Glycoprotein (NiV structural protein); HeV: Hendra Virus; HeV-sG: Hendra Virus Soluble Glycoprotein; HRC: Heptad Repeat Regions; HRP: Horseradish Peroxidase; ICMR: Indian Council of Medical Research; ICTV: International Committee on Taxonomy of Viruses; IEDCR: Institute of Epidemiology, Disease Control and Research; IFNAR-KO: Interferon Alpha/Beta Receptor Knockout; IgG: Immunoglobulin G; IgM: Immunoglobulin M; IKK: IκB Kinase; ILL: Influenza-Like Illness; IP: Intraperitoneal; IRF: Interferon Regulatory Factor; IV: Intravenous; JEV: Japanese Encephalitis Virus; L: Polymerase Protein (NiV structural protein); LC-MS/MS: Liquid Chromatography-Tandem Mass Spectrometry; LC16m8: A vaccinia virus strain; M: Matrix Protein (NiV structural protein); MDA5: Melanoma Differentiation-Associated Protein 5; MODS: Multiple Organ Dysfunction Syndrome; mAbs: Monoclonal Antibodies; mRNA: Messenger RNA; N: Nucleoprotein (NiV structural protein); NCEP: National Centre of Epidemiology, Philippines; NETs: Neutrophil Extracellular Traps; NGS: Next-Generation Sequencing; NiV: Nipah Virus; NiV-B: Nipah Virus Bangladesh Strain; NiV-M: Nipah Virus Malaysia Strain; NiVpv: Nipah Pseudovirus; NIAID: National Institute of Allergy and Infectious Diseases; P: Phosphoprotein (NiV structural protein); PEG4: Polyethylene Glycol 4; PHV02: A chimeric vaccine candidate; PL: Proximity Labeling; PPE: Personal Protective Equipment; qRT-PCR: Quantitative Reverse-Transcription Polymerase Chain Reaction; RLR: RIG-like Receptors; ROS: Reactive Oxygen Species; RT-PCR: Reverse Transcription Polymerase Chain Reaction; SERBP1: Serpine mRNA Binding Protein 1; STMN1: Stathmin 1; TBK1: TANK-Binding Kinase 1; TCID50: Tissue Culture Infectious Dose 50; TLR: Toll-like Receptor; V: Viral Protein (NiV immune evasion protein); VI: Virus Isolation; VSV: Vesicular Stomatitis Virus; W: Viral Protein (NiV immune evasion protein); WGS: Whole-Genome Sequencing; WHO: World Health Organization.
Acknowledgments
Authors acknowledge CARE for financial and infrastructural support. VK acknowledges CARE for providing the fellowship and financial support.
Author’s contributions
VK collected data and prepared the initial draft with the help of KG. AG was involved in the conception, designing, and additional data collection, analysis, and final manuscript preparation. All the listed authors approved the final draft.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

