Unraveling Common Stem Cell Sources and Key Reporting Parameters in Studies Related to Stem Cell-Derived Red Blood Cells: A Review
- Department of Clinical Medicine, Advanced Medical and Dental Institute, Universiti Sains Malaysia, 13200 Kepala Batas, Pulau Pinang, Malaysia
- Transfusion Medicine Unit, Clinical Diagnostic Laboratory, Advanced Medical and Dental Institute, Universiti Sains Malaysia
- Regenerative Medicine Cluster, Advanced Medical and Dental Institute, Universiti Sains Malaysia, 13200 Kepala Batas, Pulau Pinang, Malaysia
- Department of Biomedical Science, Advanced Medical and Dental Institute, Universiti Sains Malaysia, 13200 Kepala Batas, Pulau Pinang, Malaysia
- Department of Toxicology, Advanced Medical and Dental Institute, Universiti Sains Malaysia, 13200 Kepala Batas, Pulau Pinang, Malaysia
- Craniofacial and Biomaterial Group, Department of Dental Science, Advanced Medical and Dental Institute, Universiti Sains Malaysia, 13200 Kepala Batas Penang Malaysia
Abstract
Introduction: Blood transfusions are essential for maintaining oxygen delivery to tissues in cases of severe blood loss. However, challenges such as limited donor availability, short storage lifespans, blood-type incompatibility, and infection risks necessitate alternative solutions. Stem cell-derived red blood cell (RBC) substitutes offer a promising approach to address these limitations. Multiple stem cell sources, including embryonic stem cells (ESCs), hematopoietic stem cells (HSCs), and induced pluripotent stem cells (iPSCs), have been explored for RBC generation. ESCs pose ethical and immunogenicity concerns; HSCs exhibit limited proliferation potential and variable outcomes; iPSCs face safety, standardization, and scalability challenges for RBC generation. Despite significant research in this area, no comprehensive mapping of the evidence exists. This scoping review aims to systematically map the literature on stem cell-derived RBC substitutes and identify key reported parameters.
Methods: This study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement for Scoping Reviews (PRISMA-ScR) framework for scoping reviews, encompassing five key stages: defining research questions, identifying relevant studies, selecting eligible articles, data charting, and summarizing findings. A systematic search was conducted using the PubMed and Scopus databases. From 11,074 identified articles, 15 studies met the eligibility criteria. Extracted data focused on stem cell sources, culture conditions, RBC maturation (enucleation rate and hemoglobin composition), and expansion efficiency.
Results: The analysis revealed that ESCs were the most frequently utilized stem cell source, followed by HSCs and iPSCs. HSCs demonstrated the most favorable outcomes, with faster culture times (fewer than 21 days) and higher enucleation rates, ranging from 50% to 98% in some studies. ESCs exhibited higher RBC yields but showed lower enucleation efficiency. In contrast, iPSCs had the lowest enucleation rates, indicating challenges in their use for RBC generation. Key culture parameters, including cytokine supplementation, oxygen tension, and differentiation protocols, significantly influenced RBC yield and maturation.
Conclusion: Stem cell-derived RBCs represent a viable alternative to conventional blood transfusions, offering an unlimited source of RBCs while addressing donor-related challenges. Among the examined stem cell types, HSCs demonstrated the most promising characteristics in terms of culture efficiency and enucleation rates. This review provides a comprehensive overview of essential parameters for advancing RBC generation and serves as a valuable resource for future research in the development of stem cell-based blood substitutes.
Introduction
Blood loss compromises the body’s ability to deliver oxygen to tissues, necessitating blood transfusions to restore balance. While blood transfusion is generally a safe and life-saving practice, several challenges persist. These include the limited storage life and specific temperature requirements of donated blood, the risk of transfusion reactions due to blood-type incompatibility, the rising demand for blood as donor numbers decline, and the potential transmission of infections1, 2, 3.
To address these challenges, scientists have sought alternative solutions in the form of blood substitutes. These substitutes aim to replicate the functions of natural blood components, particularly RBCs, while overcoming the limitations of traditional blood transfusion4. Blood substitutes are broadly categorized into chemical and biological oxygen carriers. Chemical substitutes are acellular and include perfluorocarbon (PFC) and polymer-based oxygen carriers. Biological substitutes, on the other hand, include hemoglobin-based oxygen carriers (HBOCs) and RBCs derived from stem cells in the lab, which are partially or entirely composed of biological materials5.
Stem cells are undifferentiated cells generated during the early stages of embryonic development. Unlike adult cells, they lack specialized functions and phenotypic characteristics. Their unique ability to self-renew and differentiate into specific cell types has made them a cornerstone of therapeutic applications, particularly in the regeneration and repair of damaged tissues6, 7, 8. Stem cells were first discovered in the early 20 century, and by the mid-century, stem cells isolated from bone marrow were successfully transplanted, marking a significant breakthrough in medicine. These transplants were performed between related and unrelated donors to treat a range of conditions. By the late 20 century, embryonic stem cells were successfully isolated from the inner cell mass of early embryos9, 10, 11, 12. In 2006, Takahashi and Yamanaka revolutionized the field by describing induced pluripotent stem cells (iPSCs), which were derived from both embryonic and adult fibroblast cultures13, 14. Stem cells can be broadly categorized into embryonic stem cells (ESCs) and adult stem cells based on their potency and origin. ESCs, derived from the inner cell mass of blastocysts, are pluripotent and capable of differentiating into any cell type in the body. After 5–6 days of fertilization, ESCs can be isolated from the blastocyst and cultured for various applications. In contrast, adult stem cells are multipotent, residing in specific tissues such as bone marrow, liver, brain, dental pulp, and the eye. These cells remain undifferentiated and can renew themselves to produce cells specific to their tissue of origin. Hematopoietic stem cells (HSCs), found in the bone marrow, are a prime example of adult stem cells capable of differentiating into various blood cell types15, 16, 17.
Artificially generating red blood cell-like cells in vitro has been a significant area of research. ESCs, HSCs, and iPSCs have all been utilized in this context18, 19, 20. HSCs, widely used in bone marrow transplant procedures, can be harvested from bone marrow, peripheral blood, or cord blood21. Meanwhile, iPSCs, discovered by Yamanaka in 2007, are reprogrammed somatic cells that regain stem cell-like properties, offering a similar potential to ESCs14, 22. The exploration of producing red blood cells (RBCs) from stem cells began over 20 years ago. In 1998, a team led by Donald Kohn successfully developed a system to produce human RBCs from hematopoietic sources, marking the first step toward fully mature RBC production using recombinant growth factors23. Mature, enucleated RBCs were isolated and tested for their functional properties, spurring further interest in stem cell research. In 2005, Luc Douay and his team produced large-scale mature RBCs in the laboratory using hematopoietic stem cells (HSCs) and a combination of culture media, cytokines, and reagents21. By 2008, Robert Lanza's group succeeded in regenerating fully functional oxygen-carrying RBCs from human embryonic stem cells (hESCs). Their work demonstrated the cells' ability to carry oxygen efficiently, with an oxygen equilibrium curve comparable to that of natural RBCs. These cells underwent progressive maturation, including size reduction, increased glycophorin A expression, and chromatin thickening, resulting in RBCs with diameters of approximately 6 to 8 µm24.
In 2011, Douay's group further validated the functionality of lab-produced RBCs by demonstrating their survival in human circulation25. These RBCs not only bound, transported, and released oxygen but also expressed blood group antigens on their surface. When injected into a human subject, these cells displayed a half-life of 26 days, comparable to the 28 ± 2 days of native RBCs, with 63% of the injected cells remaining after 26 days of circulation21.
While large-scale production of laboratory-generated RBCs is theoretically achievable, significant challenges remain. The process is currently cost-prohibitive due to the extensive use of growth factors, cytokines, and specialized reagents required for cell culture and differentiation. Despite these limitations, research efforts continue to optimize the methods for producing RBCs. For example, Joanne Mountford and her team at the University of Glasgow have made significant contributions to regenerative medicine, exploring various progenitor stem cells, including iPSCs, HSCs, and hESCs, as alternatives to traditional transfusion products26, 27, 28.
A breakthrough came in 2017 when Trakarnsanga of the University of Bristol developed a novel approach to producing a continuous and stable supply of RBCs using immortalized early adult erythroblasts. Immortalized cell lines, which can proliferate indefinitely in vitro due to natural or induced transformation, offer a promising solution for overcoming the limitations of traditional methods29. This advancement represents a significant step toward sustainable and scalable RBC production for therapeutic applications.
The past three decades have seen remarkable advancements in the generation of RBCs from stem cell sources. Stem cells, the progenitors of specialized cells, are first observed during early embryonic development. Classified as either embryonic stem cells (ESCs) or adult stem cells, they vary in differentiation potential. ESCs are pluripotent, capable of differentiating into all cell types, whereas adult stem cells, such as hematopoietic stem cells (HSCs) found in bone marrow, are multipotent and generate specific blood cell types. Laboratory-derived RBCs have primarily been generated from ESCs, HSCs, or induced pluripotent stem cells (iPSCs)30, 31, 32, 33, 34, 35. Comparatively, ESCs, derived from early embryos, offer high pluripotency but raise ethical concerns. HSCs, obtained from adult or neonatal sources, naturally commit to blood lineages but exhibit limited expansion. iPSCs bypass major ethical issues by reprogramming adult cells yet face safety and reproducibility challenges. Each source presents unique advantages and constraints relevant to RBC generation.
In parallel, stem cell-derived red blood cells have emerged as a promising alternative, offering a potentially unlimited resource of red blood cells, particularly for rare blood groups, while mitigating risks such as infections36. Cellular-based therapies pose lower toxicity risks compared to chemical approaches and represent a revolutionary step toward regenerative medicine37, 38, 39. Stem cell technologies continue to captivate researchers, providing hope for overcoming the challenges associated with developing artificial oxygen carriers.
Several clinical trials exploring stem cell-derived red blood cells (RBCs) have emerged, particularly focusing on iPSC- and HSC-derived products due to their scalability and reduced ethical concerns. For example, the RESTORE trial (United Kingdom) has investigated lab-grown RBCs from adult stem cells for transfusion in rare blood disorders. Regulatory frameworks, such as those by the Food and Drug Administration (FDA) and European Medicines Agency (EMA), emphasize cell source traceability, GMP-compliance, and long-term safety monitoring. Publicly disclosed trials remain limited, highlighting the early phase of clinical translation and the need for harmonized regulatory guidance to accelerate therapeutic adoption40.
Despite tremendous progress in culturing RBCs , optimizing the chemical and growth factor environment remains a significant hurdle, particularly in reducing costs. Nevertheless, stem cell-derived RBCs offer promising potential for addressing the limitations of traditional transfusion practices, with researchers working tirelessly to make this vision a reality.
Currently, there is limited comprehensive evidence mapping the studies on RBCs derived from stem cell sources. This scoping review aims to highlight the three most sourced stem cell derivatives in the field of red cells studies and production. The pursuit of stem cell-derived RBCs stems from the need to address the challenges of traditional transfusion, including limited storage, compatibility issues, undetectable pathogen risks, and an aging donor population. Among the various types of blood substitutes, stem cell-derived RBCs present the greatest potential for compatibility with human biology, offering a pathway to revolutionize transfusion medicine and blood banking services.
This review focuses on ESCs, HSCs, and iPSCs as they represent the most widely studied and biologically distinct categories of stem cells used in red blood cell (RBC) generation. These sources encompass pluripotent (ESCs, iPSCs) and multipotent (HSCs) cell types, providing a comparative perspective on their differentiation potential, ethical considerations, and translational readiness. Immortalized erythroid cell lines, while promising for large-scale RBC production, are typically derived from these stem cell types and fall outside the primary scope of this review, which aims to evaluate the foundational stem cell sources and the key parameters reported in their use for RBC generation. Future reviews may more specifically explore immortalized cell lines as a distinct category of interest.
Methods
The review was conducted in accordance with the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement for Scoping Reviews (PRISMA-ScR)41.
Search Strategy
A comprehensive literature search was carried out using the PubMed and Scopus databases to identify relevant studies from January to June 2022. These databases were selected for this review because of their broad biomedical coverage, strong indexing of peer-reviewed journals, and user-friendly interfaces, ensuring efficient and high-quality literature retrieval within time and personnel constraints. However, we acknowledge that excluding additional databases such as EMBASE and Web of Science may introduce selection bias and could result in the omission of relevant studies indexed exclusively there. Future reviews may benefit from a broader database strategy to enhance comprehensiveness and reduce the risk of publication bias. Nonetheless, the search strategy used a combination of keywords related to "stem cells," "embryonic stem cells," “hematopoietic stem cells,” "induced pluripotent stem cells," “red cell substitute,” “red blood cells,” “oxygen carrier,” “blood production,” “blood substitutes,” and “artificial blood.” No other search strings beyond the determined keywords were employed.
Study Selection
The search aimed to identify research exploring key parameters of different stem cell sources used in stem cell-derived red blood cells.
Eligibility
This review included original research articles, clinical trials, in vitro studies, and animal model studies exploring stem cell-derived red blood cells as an alternative to conventionally procured blood in transfusion medicine and blood bank services. Only English-language studies were considered. Exclusion criteria included studies that did not specifically focus on blood substitutes, those unrelated to stem cell-derived red blood cells, or research centered on non-stem cell approaches. The exclusion criteria also encompassed non-scientific articles, studies published in languages other than English, and research published before 1990 or after June 2022.
In addition to exclusion based on relevance, duplicates, incomplete data, commentary pieces, conference abstracts without full manuscripts, and non-peer-reviewed sources were also excluded to maintain data quality and consistency. Some potentially relevant articles were excluded due to language barriers or lack of full-text access. Non-English studies were excluded primarily due to translation limitations and the lack of reliable linguistic resources, which could have compromised accurate data extraction and interpretation. We acknowledge that this introduces a potential language bias, possibly omitting valuable insights published in other languages. To mitigate this, we thoroughly screened the titles and abstracts across the two selected databases (PubMed and Scopus), known for indexing a broad spectrum of high-impact, peer-reviewed English-language publications. Nonetheless, future reviews could benefit from multilingual collaboration to enhance inclusivity and comprehensiveness.
Both qualitative and quantitative studies were considered. Qualitative research included experience reports, literature reviews, integrative reviews, systematic reviews, meta-analyses, and scoping reviews. For quantitative studies, case-control, prospective and retrospective cohort, and experimental studies were included. Additionally, relevant citations and references from all identified studies were reviewed to ensure comprehensive coverage.
The screening and selection process was conducted using Mendeley version 1.19.4 reference management software, following a structured three-phase approach. In Phase I, duplicate records were removed. Phase II involved screening article titles and abstracts, while Phase III entailed a comprehensive review of the full-text articles selected from the previous phase. Three independent reviewers (BHOAA, SSN, and SNFMN) assessed the titles and abstracts of all identified studies. To ensure reliability and minimize bias, they followed a standardized selection and data extraction protocol. Any discrepancies during study selection or data extraction were resolved through discussion among the reviewers. If disagreements remained unresolved, a fourth reviewer (MAZ) was consulted to facilitate consensus. In cases where consensus could not be reached immediately, the study was re-evaluated collectively before a final decision was made. This process ensured consistency, minimized bias, and maintained the integrity of the review.
Data Extraction and Analysis
For each included study, a structured data extraction process was employed to collect key information, including author, publication year, stem cell type, culture period, study purpose, and parameters of RBC maturation (such as enucleation rate and expansion yield). To ensure accuracy and consistency, the reviewers collaboratively examined the extracted data, minimizing variability in interpretation.
Additionally, to enhance transparency and reproducibility, the standardized data extraction form used in this review has been provided as supplementary material (Supplementary 1). The form includes key fields such as stem cell type, source, culture conditions, differentiation protocols, enucleation rates, hemoglobin expression, and study outcomes, which were consistently used across all included studies.
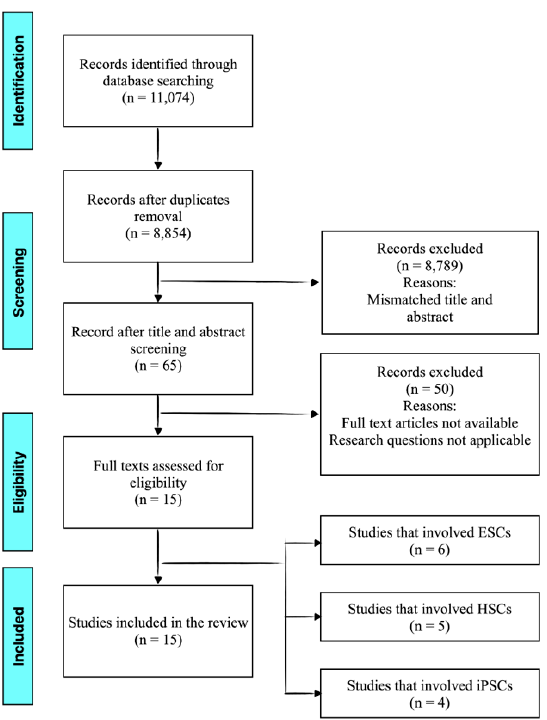
This review presents its findings using a narrative synthesis approach, adhering to the PRISMA-ScR guidelines to comprehensively identify all available evidence and highlight key characteristics. Figure 1 provides an overview of the literature search process. Initially, 11,074 articles were extracted from PubMed and Scopus. After removing duplicates, 8,854 articles remained. Sixty-five articles were included after removing titles and abstracts that did not meet the screening criteria. Next, three reviewers read these articles to confirm whether each one satisfied the research questions and eligibility criteria.
Finally, 15 full-text articles were included and subsequently subclassified based on stem cell type: six studies on ESCs, five on HSCs, and four on iPSCs. ESC-related studies predominated in this scoping review, whereas HSCs and iPSCs had an equal number of studies examining the most suitable stem cell-derived RBCs.

Study selection flowchart. From 11,074 records identified, 8,854 remained after removing duplicates. After screening titles and abstracts, 65 articles were reviewed, with 15 full texts assessed. In total, 15 studies were included: six on ESCs, five on HSCs, and four on iPSCs.
Characteristics of included studies
|
Author (Year) |
Type of stem cell & Culture time |
Aim |
RBCs maturation |
Yield/expansion (RBCs per stem cell blood unit) | |
|---|---|---|---|---|---|
|
Enucleation rate (%) |
Hb ration (Hb expression) | ||||
|
Chang |
hESCs 15 to 56 days |
To study the expressed Hb in RBCs-like cells from hESCs |
Not quantified |
Embryonic Hb Gower I (ζ2ε2) HbF (γ2) Rare HbA (α and β globins) |
Not quantified |
|
Lu |
hESCs 19-21 days |
To study the biological properties of in lab enucleated RBCs from hESCs. |
> 60% |
Embryonic Hb Gower I (ζ2ε2) HbF (α2γ2) HbA, only some α chains |
3.86 x 1010 |
|
Hiroyama |
murine ESCs 120 days |
To develop functional RBCs from murine ESCs |
Not quantified |
Only HbA (α and β globins) |
Visible, improved RBC counts |
|
Ma |
hESCs 18 days |
To develop functional erythrocytes from hESCs |
> 60 to 82% |
γ and β globins |
2 × 104 - 106 |
|
Honig (Lanza team) |
hESCs 21 days |
To investigate the Hb subunits of erythroid cells derived from hESCs |
NA |
Hb Gower I>Hb Barts (γ4) |
Not quantified |
|
Dias |
hESCs 90 days |
To generate RBCs from hiPSCs |
12% |
ε - and γ- globins |
2.5 x 105 |
|
Malik |
HSCs 21 days |
To produce human RBCs model form HSCs |
10 to 42% |
β globins with insignificant fetal globin |
Not quantified |
|
Neildez-Nguyen |
HSCs 18 - 21 days |
To produce a large-scale |
60 to 95% |
more HbF ( HbA (> 95% |
5 x 109-2.8 x 1011 |
|
Giarratana |
HSCs 15 to 18 days |
To generate a full mature human RBCs from HSCs |
98% ±1 |
HbA > HbF (peripheral blood & adult bone marrow) HbF> HbA (cord blood) |
up to 1.95 x 106 |
|
Shah |
HSCs 18 days |
To generate and evaluate stem-derived RBCs from HSCs |
53.4% |
γ > α globins |
4 x 109 |
|
Zhang |
HSCs 21 days |
To generate large scale RBCs from HSCs |
50% ± 5.7 |
HbF and β globins |
2.9 ~5 ×1011 |
|
Lapillone (Douay group) |
hiPSCs 25 days |
To generate and differentiate hiPSCs into definitive RBCs |
4 to 10% |
ε - and γ- globins |
up to 4.4 x 108 |
|
Dias |
hiPSCs 90 days |
To generate RBCs from hiPSCs |
2 to 10% |
ε - and γ- globins |
6 x 108 |
|
Kobari (Douay group) |
hiPSCs 52 days |
To generate a terminal matured RBC from iPSCs |
20 to 26% |
ε - and γ globins |
1.5 - 2.8 x 109 |
|
Park |
hiPSCs 31 days |
To develop a bankable hiPSCs |
Not quantified |
Undetectable amount of HbA |
8 x 106-1.8 x 107 |
Comparative summary of all the stem cell types across same parameters included in the studies
|
Parameter |
hESCs |
HSCs |
iPSCs |
|---|---|---|---|
|
Culture Time (day) |
15 - 90 days (for murine ESCs up to 120 days) |
15 - 21 days |
25 - 90 days |
|
RBCs Yield (per stem cell blood unit) |
105 - 1010 |
106 - 1011 |
105 - 109 |
|
Enucleation Rate (%) |
>60% |
10 - 98% |
2 - 26% |
|
Hb Expression |
Mainly embryonic/HbF |
Mostly HbA |
Primarily HbF with some HbA |
Results
The final 15 full-text articles included in the final analysis were further subclassified based on the type of stem cells studied. This included six studies focused on embryonic stem cells (ESCs), five on hematopoietic stem cells (HSCs), and four on induced pluripotent stem cells (iPSCs). Studies on ESCs were the most predominant in this scoping review, while HSCs and iPSCs had an equal number of studies, reflecting ongoing efforts to identify the most suitable stem cell-derived substitutes for red blood cells.
Most of the articles identified and included in this review utilized human ESCs for culturing, with culture durations ranging from 15 to 28 days on average. However, outlier studies for human ESCs by Dias . (2011) and Chang . (2006) reported a much longer culture period of up to 56 and 90 days, respectively, while Hiroyama . (2008) reported 90 days for murine ESCs42, 43, 44. Some studies such as Chang . (2006), Hiroyama . (2008), and Honig . (2010) lacked key information, such as RBC yield and enucleation rates43, 44, 45. Such key data were either not reported or only partially described, resulting in NA (not available) entries in
Among the remaining studies, Lu . (2008)24 and Ma . (2008)46 reported enucleation rates exceeding 60%, with Lu . (2008)24 also achieving the highest yield, followed by Dias (2011)42. All studies demonstrated that progenitor cells expressed embryonic and fetal hemoglobin subunits during the differentiation process. Notably, Nakamura uniquely detected adult hemoglobin (HbA) in their cultured cells.
The study by Malik (1998) successfully established an in vitro human RBC model23. However, the enucleation rate was ≤ 42%, and yield data were not provided. The RBCs produced primarily expressed adult β-globin hemoglobin. Subsequent studies reported more promising results, with Neildez-Nguyen . (2002)47 and Giarratana . (2005)21 achieving enucleation rates exceeding 95%. More recent studies, including those by Shah . (2016)48 and Zhang . (2017)49, reported enucleation rates of 53.4% and 50%, respectively. Zhang (2017)49 also achieved the highest cell expansion rate, reaching 4.8–5 × 10¹⁹ RBCs per stem cell blood unit. While most studies reported fetal hemoglobin (HbF) as the predominant expressed form, studies utilizing peripheral blood or adult bone marrow sources21 and approaches47 demonstrated higher expression of HbA.
Notably, the outcomes of HSC-derived red blood cell production show variability across studies. For example, Malik . (1998) reported enucleation rates ranging from 10–40%, whereas Giarratana (2005) achieved up to 98% enucleation. This discrepancy may be attributed to several factors, including differences in HSC sources (, cord blood . peripheral blood), culture duration, cytokine cocktails, and stage-specific supplementation with factors such as erythropoietin (EPO), stem cell factor (SCF), or glucocorticoids. Furthermore, advances in protocol optimization and improvements in cell sorting or feeder layer techniques over time may also explain such enhanced outcomes in later studies. This underscores the importance of standardized methodologies and comprehensive reporting to ensure reproducibility and comparability across research efforts.
Lastly, among the four studies that used iPSCs, Kobari (2012)50 achieved the highest cell expansion rate, ranging from 1.5 to 2.8 × 10⁹ RBCs per stem cell blood unit. However, enucleation rates across all studies were low, not exceeding 26%. Dias . (2011)42 and Hiroyama. (2008) reported a significantly longer culture period compared to other studies, including Lapillonne (2010), Kobari (2012), and Park (2020)34, 44, 50, 51. While all studies documented the expression of embryonic and fetal hemoglobin subunits, Park . (2020), despite being the most recent, did not report the enucleation rate51.
Discussion
Analyses of the findings demonstrate the development of reticulocytes from embryonic stem cells (ESCs), hematopoietic stem cells (HSCs), and induced pluripotent stem cells (iPSCs) in laboratory settings. These studies also reported relevant process parameters. This scoping review identified key benchmarks and insights into the field. Among the stem cell types analyzed, ESCs, HSCs, and iPSCs were chosen as they are the most sourced in generating stem cell-derived red blood cells (RBCs). Other approaches, such as immortalized stem cell lineages, represent newer methods in this area but were less represented in the screened literature. For example, a research group led by Trakarnsanga at the University of Bristol is working on immortalized stem cell lineages as potential oxygen carriers30. Since 2017, this team has been conducting clinical trials in the United Kingdom with 20 to 25 human subjects under a consortium that includes the National Health Service (NHS), universities, blood service centers, and corporations. Although the type of stem cells used in these trials has not been disclosed, their work underscores the potential of alternative stem cell sources for RBC production52.
While several reviews have explored the generation of red blood cells (RBCs) from various stem cell sources, most focus either on a single cell type or broadly summarize outcomes without consolidating critical biological parameters. This scoping review addresses that gap by uniquely synthesizing and directly comparing key functional metrics across embryonic stem cells (ESCs), hematopoietic stem cells (HSCs), and induced pluripotent stem cells (iPSCs). Specifically, we analyze and map enucleation rates, hemoglobin subtype expression (HbA, HbF), culture duration, and RBC yield—four parameters that are essential for assessing the translational potential of stem cell-derived RBCs. This comparative approach not only highlights the relative advantages and limitations of each stem cell type but also serves as a foundational reference to guide optimization efforts and standardization in future research and clinical translation.
Among the three stem cell types reviewed, ESCs were the most represented in the final articles and initial screenings. This prominence is likely due to the earlier discovery of ESCs in 1981, which made them the first isolated stem cell type. Their pluripotent nature, which allows differentiation into all tissue types, and their relative ease of genetic manipulation for developing animal models have established ESCs as a key component of stem cell research. In contrast, iPSCs, discovered in 2006 by Takahashi and Yamanaka14, represent a breakthrough that was built upon insights from ESC research. HSCs, as the natural progenitors of all blood cells, offer distinct advantages due to their self-renewal capabilities and fewer ethical concerns compared to ESCs.
The use of ESCs remains ethically contentious in many jurisdictions due to their embryonic origin, leading to stricter regulatory oversight and limited public acceptance. In contrast, iPSCs and adult-derived HSCs offer more ethically acceptable and practically feasible alternatives. iPSCs provide the advantage of patient-specific compatibility and circumvent immune rejection, although their genomic stability and safety still require careful validation. Meanwhile, HSCs sourced from bone marrow, cord blood, or mobilized peripheral blood are already well-established in clinical practice, making them attractive candidates despite their lower expansion capacity.
The culture duration for transforming stem cells into enucleated RBCs is consistently reported to range between 20 and 28 days, aligning with established timelines in prior research. However, exceptions were noted, such as the work by Dias (2011)42, which required a longer culture period due to insufficient enucleation rates after RBC expansion. This review also identified a correlation between enucleation rate and yield, wherein a low or absent enucleation rate led to limited or unreported yields. Factors contributing to this limitation may include intrinsic properties of the stem cell lines, such as genetic mutations, signaling pathways, and cell membrane receptor profiles, as well as external environmental conditions, including chemical concentrations and treatment protocols. Dias . (2011) highlighted the need for further investigation into these factors42. While earlier studies, such as Malik . (1998), focused primarily on demonstrating in vitro RBC production from HSCs, they did not address large-scale production or yield but successfully reported adult β-globin expression in enucleated RBCs23.
Regarding hemoglobin expression, early studies predominantly identified embryonic and fetal hemoglobin (HbF) chains in stem cell-derived RBCs. However, Giarratana demonstrated that HSCs derived from peripheral blood and bone marrow expressed predominantly adult hemoglobin (HbA), whereas cord blood-derived RBCs exhibited higher fetal hemoglobin (HbF) levels, consistent with their natural composition21. Similarly, Neildez-Nguyen observed increased HbA expression when laboratory-derived RBCs were introduced into a physiological environment, suggesting that biophysical factors and chemical interactions can influence hemoglobin expression profiles47.
In addition to these parameters, researchers should consider reporting other critical characteristics, including biochemical and membrane properties (., 2,3-diphosphoglycerate and ATP levels), O2-Hb dissociation curves, cell size (compared to the normal RBC diameter of 6–8 µm), hemoglobin content, estimated transfusion units relative to therapeutic RBC requirements (approximately 2 × 10¹² RBCs per stem cell blood unit), and whether laboratory-derived RBCs express specific blood group antigens or resemble universal O Rh-negative RBCs.
Based on the results analyzed earlier, HSCs appear to be the most promising source for generating RBC substitutes. They demonstrate robust erythroid maturation within a consistent culture period of 21 days, achieving high yields with favorable enucleation rates. Additionally, HSCs are associated with fewer ethical concerns compared to ESCs and are less costly than iPSCs. Among HSC sources, discarded cord blood units, which often fail to meet volume thresholds for transplantation, represent an underutilized resource for RBC expansion. Unfortunately, these units are not usually stored for this purpose.
Some HSC-related studies demonstrate suboptimal outcomes in terms of red blood cell yield, enucleation rates, or hemoglobin expression. This underperformance can be attributed primarily to source heterogeneity and protocol variability. HSCs can be derived from various sources such as bone marrow, peripheral blood, or umbilical cord blood, each with distinct biological characteristics and proliferative capacities. For instance, cord blood-derived HSCs may exhibit higher proliferation but lower enucleation efficiency compared to adult sources. Additionally, differences in isolation techniques, such as CD34 selection purity, as well as variability in cytokine combinations, serum supplementation, and oxygen tension during culture, significantly impact differentiation outcomes. Early-phase studies may also lack stage-specific optimization of differentiation protocols or use outdated culture systems, leading to inconsistent or inefficient RBC production. These disparities highlight the urgent need for harmonized protocols and more precise characterization of starting HSC populations to improve reproducibility and clinical translation.
Finally, future research should focus on addressing the key bottlenecks in scaling up stem cell-derived RBC production, including cost-effectiveness, manufacturing efficiency, and batch-to-batch reproducibility. A major hurdle in clinical translation is the low RBC yield per stem cell unit relative to transfusion needs. Current protocols often generate insufficient quantities, far below the ~2×10¹² RBCs required for a single unit of transfusable blood. Achieving such volumes would require substantial upscaling, optimized bioreactor systems, and cost-effective, serum-free media, all of which remain ongoing challenges in the field.
Moreover, current differentiation protocols often involve costly cytokines and prolonged culture times, which limit clinical and commercial viability. Developing serum-free, chemically defined media and integrating bioreactor-based expansion systems could help streamline production. Furthermore, advances in gene editing and synthetic biology may enhance enucleation efficiency and hemoglobin switching to adult forms, further improving the functionality of lab-generated RBCs.
Although notable protocol differences across studies were identified, we did not conduct a meta-regression analysis due to the substantial heterogeneity in study designs, outcome reporting, and the small number of eligible studies. As a scoping review, our primary objective was to map existing evidence and summarize key parameters, rather than statistically pool outcomes. Given these limitations, a qualitative synthesis was deemed more appropriate. However, future systematic reviews with a larger, more standardized dataset could explore quantitative approaches such as meta-regression to better assess the impact of protocol variability on outcomes like enucleation rate, yield, and hemoglobin expression.
In summary, this review provides strong evidence supporting the feasibility of generating RBCs from various stem cell sources, with HSCs emerging as the most viable option for future applications. However, the review also highlights a lack of comprehensive studies that address all critical parameters for stem cell-derived RBC production. This gap is partly due to variations in protocols and methodologies, as well as challenges during the initial screening process, which yielded unrelated results due to shared keywords with other biomedical fields. Despite these limitations, the findings underscore the significant potential of stem cell-derived RBCs as substitutes for transfusion purposes, while also identifying areas that require further research and standardization.
Conclusions
In conclusion, significant and rapid progress has been made in the development of blood transfusion alternatives. This scoping review highlights that ESCs are the most widely used stem cell source for generating RBC substitutes, followed by HSCs and iPSCs. These three stem cell types have emerged as the primary candidates for artificial blood production. The review aims to assist researchers by providing essential insights into key parameters that should be considered when reporting and publishing experimental findings on artificial blood, including enucleation rate, hemoglobin composition, and cell expansion.
Based on the findings, HSCs appear to be the most promising source for blood cell substitutes, demonstrating superior culture efficiency, enucleation rates, and expansion potential compared to other stem cell types. While HSCs demonstrate favorable outcomes in terms of culture duration and enucleation efficiency, it is important to note that they represent a heterogeneous population derived from various sources, including cord blood, bone marrow, and mobilized peripheral blood. These sources differ in proliferation capacity, differentiation potential, and accessibility, which may significantly influence the reported outcomes. Therefore, while HSCs appear promising, their performance cannot be universally generalized without accounting for source-specific variations. Future studies should stratify findings based on HSC origin to enable more precise comparisons and optimize protocols accordingly. Nonetheless, this review serves as a valuable resource for scientists by summarizing previous studies and their key findings, offering a comprehensive and concise reference to support future research. Additionally, it encompasses studies focusing on RBC substitutes derived from ESCs, HSCs, and iPSCs.
Emerging approaches such as immortalized erythroid progenitor cell lines offer a promising avenue for consistent, large-scale production of red blood cells with higher yields and improved reproducibility. These cell lines bypass donor variability and enable standardized, controlled erythropoiesis under GMP conditions. While not the primary focus of this review, such technologies could complement stem cell-based methods by providing stable platforms for preclinical testing and transfusion applications.
Looking forward, future studies should aim to adopt standardized reporting frameworks that include details on cell source, passage number, culture conditions, enucleation efficiency, hemoglobin subtype expression, and yield per input cell. Transparent, harmonized reporting will facilitate cross-study comparison, replication, and eventual clinical translation of stem cell-derived RBC technologies.
However, the final number of included articles was limited due to restrictions in full-text availability and language barriers. The scope of databases used in this review may also have led to the unintentional omission of relevant studies on stem cell-derived blood substitutes. Furthermore, as this review did not include a formal quality appraisal assessment, some lower-quality studies may have been included.
While this review aimed to comprehensively capture relevant studies on stem cell-derived red blood cells, some publications were excluded due to full-text unavailability or language constraints. This may introduce a degree of selection bias, as potentially valuable data particularly from non-English-speaking regions could not be assessed. Acknowledging this, future reviews could benefit from multilingual collaboration or expanded database access to enhance global representation and comprehensiveness of the evidence base. Despite these limitations, this study provides a valuable foundation for advancing research in artificial blood substitutes and highlights key areas for further investigation.
Abbreviations
ATP: Adenosine Triphosphate, CD34+: Cluster of Differentiation 34, EMA: European Medicines Agency, EPO: Erythropoietin, ESC / ESCs: Embryonic Stem Cell(s), FDA: Food and Drug Administration, GMP: Good Manufacturing Practice, HBOCs: Hemoglobin-Based Oxygen Carriers, HbA: Adult Hemoglobin, HbF: Fetal Hemoglobin, HSC / HSCs: Hematopoietic Stem Cell(s), IL-3: Interleukin-3, IMDM: Iscove’s Modified Dulbecco’s Medium, iPSC / iPSCs: Induced Pluripotent Stem Cell(s), NHS: National Health Service, PFC: Perfluorocarbon, PRISMA-ScR: Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement for Scoping Reviews, RBC / RBCs: Red Blood Cell(s), RESTORE (trial name, not spelled out in the text), SCF: Stem Cell Factor.
Acknowledgments
We sincerely thank the Ministry of Higher Education, Malaysia, for supporting this research through the Fundamental Research Grant Scheme (FRGS), which facilitated the work presented in this scoping review.
Author’s contributions
(I) Conceptualization, methodology development, data curation, formal analysis, and drafting of the original manuscript: MAZ, SSN (II) Administrative support, critical review, and manuscript editing: BHOAA, SSN, SNFMN, MYA, RM (III) Data acquisition, validation, and analysis: MAZ, BHOAA, SSN (IV) Experimental execution and dataset compilation: BHOAA, MYA, RM (V) Data analysis, interpretation, and result evaluation: MAZ, BHOAA, SSN, SNFMN (VI) Final approval of the manuscript and accountability for the integrity of the work: All authors.
Funding
This review is a part of research funded by the Fundamental Research Grant Scheme (FRGS), Malaysia [Grant number: FRGS/1/2019/STG07/USM/02/13].
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

