TLR4 Agonist CRX-527 Modulates Intracellular and Inflammatory Cytokine Expression in Lymphoid Tissues of BCG-MSP1C-Immunized Mice
- School of Health Sciences, Health Campus, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia
Abstract
Introduction: Due to the complexity of the malaria parasite’s life cycle, together with the limited knowledge of its intricate immunological response, this study was conducted. This study aims to evaluate the ability of a Toll-like receptor 4 (TLR4) agonist, CRX-527, to enhance the production of intracellular and inflammatory cytokines in lymphocyte-derived tissues of malaria vaccine candidates.
Methods: A total of forty-eight male Balb/c mice was randomly divided into eight groups (n = 6 per group). Two groups of mice were immunized intraperitoneally with 2 × 106 colony-forming units (CFU) of parent Bacillus Calmette–Guérin (BCG) in 200 µl of 1 × phosphate-buffered saline–Tween 80 (PBS-T80) in the presence or absence of 0.5 mg/kg of CRX-527. Another two groups of mice were immunized with 2 × 106 CFU of Mycobacterium bovis Bacillus Calmette–Guérin expressing the C-terminus merozoite surface protein-1 of Plasmodium falciparum (BCG-MSP1C) in 200 µl of 1 × PBS-T80 in the presence or absence of 0.5 mg/kg of CRX-527. Meanwhile, another two groups of mice were immunized with 200 µl of 1 × PBS-T80, whereas the remaining two groups were immunized with 200 µl of lipopolysaccharide (LPS) in the presence or absence of 0.5 mg/kg of CRX-527, serving as control groups. The lymphoid tissues obtained from the spleen, lymph nodes, and liver were harvested from the euthanized mice and were cultured for 24 hours. Then, the supernatant was collected to detect the production of selected intracellular and inflammatory cytokines using a sandwich enzyme-linked immunosorbent assay (ELISA).
Results: There was a significant increase (p < 0.05) in tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), interferon-gamma (IFN-γ), and interleukin-4 (IL-4) in the presence of CRX-527, with the highest levels observed in the spleen, followed by the lymph nodes and liver from the BCG-MSP1C groups, followed by the parent BCG-immunized groups.
Conclusion: These findings demonstrate that the increased production of several intracellular and inflammatory cytokines by the developed malaria vaccine candidate (BCG-MSP1C) in the presence of CRX-527 plays an important role in triggering innate immune responses in lymphocyte-derived tissues.
Introduction
Malaria is a significant and widespread infectious disease caused by parasites of the Plasmodium genus, which has contributed to a major public health problem in many developing countries, especially in tropical and subtropical regions1. Despite numerous initiatives conducted for malaria prevention and treatment, there is still a huge demand for an effective malaria vaccine due to the emergence of drug-resistant malaria parasites and the limited success of existing vaccines2. The primary pathophysiological events in malaria infection include (a) the destruction of red blood cells and impaired red blood cell production, (b) the adhesion of Plasmodium-infected red blood cells to capillaries in vital organs, and (c) the excessive production and release of proinflammatory cytokines such as IFN-γ, TNF-α, and interleukins3.
Various methods have been explored to deliver malaria vaccines to the immune system. Previously, our laboratory developed a recombinant BCG clone containing a synthetic gene of the C-terminus merozoite surface protein-1 of (BCG-MSP1C)4. A study by Mohammad showed that this clone stimulated higher cellular and humoral immune responses in animals and was capable of stimulating phagocytic activity and pro-inflammatory cytokine production in mouse and human macrophages5. The clone can also trigger the macrophage inflammatory response by activating TLR46, suggesting the possible use of TLR4 as an adjuvant to enhance a long-term immune response to a potential malaria vaccine. Toll-Like Receptors (TLRs) are receptors involved in the innate immune response, playing a crucial role in recognizing pathogens and initiating inflammatory responses7. TLR4 agonists are molecules that activate TLR4, triggering its signaling pathway to initiate immune responses. TLR4 also interacts with intracellular ligands to start complex intracellular signaling cascades, in addition to engaging with foreign ligands at the surface of cellular membranes8. When TLR4 is over-stimulated, it can lead to excessive inflammation and unfavorable outcomes in viral infections9, 10.
On the other hand, TLR4 agonists can be used to enhance the innate immune response and promote inflammation in certain therapeutic contexts, such as vaccine adjuvants or cancer immunotherapy11. TLR4 agonists have been studied for their potential in treating infectious diseases and cancers by boosting the immune response. CRX-527 is one of the most potent TLR4 agonists and has been chosen as an optimized adjuvant for different disease models12, 13. However, until recently, the lack of knowledge regarding the mechanisms behind human adjuvant formation has hindered the development of vaccines and cancer immunotherapy. This study aims to evaluate the ability of the TLR4 agonist CRX-527 to enhance the production of several intracellular and inflammatory cytokines in lymphocyte-derived tissues in response to the BCG-MSP1C vaccine. The production of selected intracellular and inflammatory cytokines, with or without CRX-527, was also analyzed.
Methods
BCG and rBCG Cultures
A recombinant BCG (rBCG), BCG-MSP1C, and parent BCG (Japan) were cultured separately on 7H11 agar (Becton Dickinson, Franklin Lakes, New Jersey, USA) which was supplemented with oleic acid, albumin, dextrose, and catalase (OADC) (Becton Dickinson, Franklin Lakes, New Jersey, USA). Previously, the rBCG was constructed in the lab from Mycobacterium bovis BCG that expressed a synthetic gene encoding MSP-1C of 14. For the BCG-MSP1C culture, 15 micrograms per milliliter (µg/ml) of kanamycin was added to the agar. The colonies of each culture were then transferred to flasks containing 10 milliliters (ml) of 7H9 broth (Becton Dickinson, USA) supplemented with OADC, with a repeated addition of 15 µg/ml of kanamycin for the BCG-MSP1C culture. All cultures were observed for any signs of contamination for the next 2–3 weeks until the optical density (OD) was approximately 0.8 (A600 ≈ 0.8). For BCG and BCG-MSP1C cultures, the colony-forming unit was computed so that 0.1 OD = 4 × 10 colony-forming units per milliliter (CFU/mL), according to Abdikarim .15.
Animal and Ethics
Forty-eight mice, which were obtained from the Animal Research and Service Centre (ARASC), USM, were used in this study. The mice were housed in ARASC and provided with sufficient food and water. The animal work was conducted with the approval of the Universiti Sains Malaysia (USM) Animal Ethics Committee, USM/IACUC/2020/(125) (1099).
Immunization of Mice
A total of forty-eight male mice weighing 20–30 grams (4–6 weeks of age) were randomly divided into eight groups (n = 6 per group). The sample size was designed according to a previous study of Abbas & Rapeah6. Two groups of mice were intraperitoneally immunized with 2 × 10 CFU of parent BCG in the presence or absence of 0.5 mg/kg of CRX-527. Another two groups of mice were immunized with 2 × 10 CFU of BCG-MSP1C in the presence or absence of 0.5 mg/kg of CRX-527. These antigen solutions were prepared according to a study by Abbas & Rapeah6. Meanwhile, another two groups of mice were immunized with 200 µl of 1× PBS-T80 but in the presence or absence of 0.5 mg/kg of CRX-527, and another two groups of mice were immunized with 200 µl of LPS in the presence or absence of 0.5 mg/kg of CRX-527 as the control groups.
Immunization of
|
Immunization of | |||
|
Control (PBS-T80) |
Positive Control (LPS) |
Treatment I (2 X 106 CFU BCG) |
Treatment II (2 X 106 CFU BCG-MSP1C) |
|
1) PBS-T80 (n = 6) |
1) LPS (n = 6) |
1) BCG (n = 6) |
1) BCG-MSP1C (n = 6) |
|
2) PBS-T80 + CRX-527 (n = 6) |
2) LPS + CRX-527 (n = 6) |
2) BCG + CRX-527 (n = 6) |
2) BCG-MSP1C + CRX-526 (n = 6) |
Lymphocytes Isolation
Four weeks after the final booster, all mice were humanely sacrificed via anesthesia with 100 mg/kg of sodium pentobarbital administered intraperitoneally. The lymphoid tissues were harvested from the spleen, lymph nodes, and liver of the euthanized mice, as these tissues are involved in the immune response to malaria parasites. The isolated tissues were cut into small pieces and placed on ice-cold RPMI 1640 medium. The digested tissue fragments were pressed through a 70 µm cell strainer and suspended in Roswell Park Memorial Institute 1640 (RPMI 1640) medium. The lymphocytes were isolated on ice using blood cell lysis buffer, ammonium–chloride–potassium (ACK) lysis buffer. Then, the lysis solutions were centrifuged at 200 × g for 10 mins, and the cells were washed twice with 2 ml RPMI 1640 and resuspended in 1 ml complete RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin.
Cell Cultures and Measurement of Intracellular and Inflammatory Cytokines
The cell suspension was added into 8 ml of complete RPMI 1640 medium, cultured in a 25 cm³ flask, and incubated at 37°C in a carbon dioxide (CO₂) incubator (Esco Micro, Malaysia). After 24 hours, the cells were viewed under a microscope (Leica Microsystems GmbH, Germany). Cells were collected by scraping the internal surface of the flask using a cell scraper. All cells were transferred to a 15 ml tube and were centrifuged at 1500 × g for 10 mins at room temperature. Finally, the supernatant was collected and transferred into a 1.5 ml microcentrifuge tube for cytokine determination. The production of intracellular and inflammatory cytokines such as TNF-α, IL-1β, IFN-γ, and IL-4 was determined using BioLegend ELISA Max Deluxe (BioLegend, USA). Briefly, 100 µl of lymphocyte culture, as well as a standard, were added to each well of the pre-coated ELISA plates for TNF-α, IL-1β, IFN-γ, and IL-4 and were incubated at room temperature for 2 hours, followed by 5 washes. Then, 100 µl of primary antibodies (anti-mouse TNF-α, IL-1β, IFN-γ, and IL-4) was added, and the plates were incubated for 1 hour at room temperature. After 5 more washes, 100 µl of avidin horseradish peroxidase (Avidin HRP) was added, followed by a 30-minute incubation at room temperature and another 5 washes. The substrate solution was added, and the plates were incubated at room temperature for 15 minutes in the dark. Finally, a stop solution was added, and the plates were read at 450 nm using an ELISA microplate reader (Thermo Fisher Scientific, USA).
Statistical Analysis
The data were statistically analyzed using IBM Statistical Package for the Social Sciences (SPSS) Statistics ver. 27 (IBM Corp., USA). The mean values among groups were analyzed using ANOVA followed by the Bonferroni post-hoc test. The comparison of the immunized mice in the presence or absence of CRX-527 was expressed as mean ± standard error of the mean (SEM). The level of significance was set at P < 0.05.
Results
Measurement of TNF-α Productions
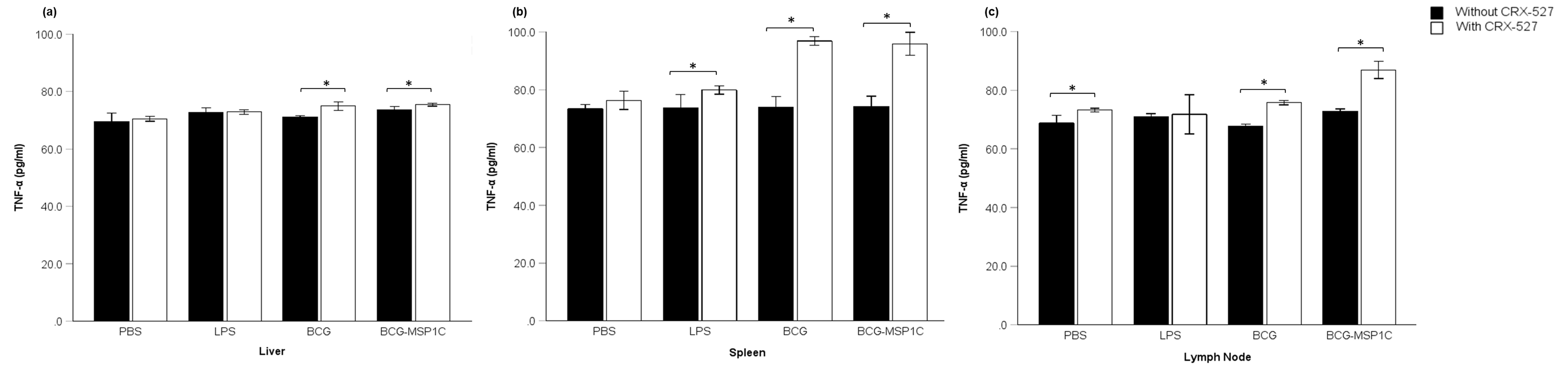
TNF-α levels in lymphocyte-derived tissues from mice immunized with BCG-MSP1C, the parent BCG, LPS, or PBS-T80, in the presence or absence of CRX 527, were measured in order to assess the influence of the TLR4 agonist on TNF-α production. Overall, the expression of TNF-α, which was analysed from the cultured supernatants using ELISA, was found to be greater in all groups with the presence of CRX 527 (Figure 1). The highest significant increase of TNF-α in the presence of CRX 527 was detected in the BCG-MSP1C-immunised groups derived from the spleen (96.92 ± 0.62 picograms per millilitre (pg/ml)) (P = 0.000), followed by the lymph node (86.89 ± 0.68 pg/ml) (P = 0.000) and the liver (75.44 ± 0.12 pg/ml) (P = 0.039). Secondly, a significant increase was detected in the BCG + CRX 527-immunised group, highest in the spleen (96.44 ± 0.27 pg/ml) (P = 0.000), followed by the lymph node (75.73 ± 0.17 pg/ml) (P = 0.000) and the liver (74.97 ± 0.34 pg/ml) (P = 0.000). A non-significant trend was detected in the LPS + CRX 527- and PBS-T80 + CRX 527-immunised groups.

Expression of TNF-α in lymphocytes-derived tissue. (a) Liver, (b) Spleen and (c) Lymph node of mice immunised with PBS-T80, LPS-T80, BCG or BCG-MSP1C in the absence or presence of TLR4 agonist, CRX 527. The concentration of TNF-α were presented in mean ± SEM. Sample size, n = 6 per group with 95% confidence interval.
Measurement of IL-1β Productions
IL-1β levels in lymphocyte-derived tissues from mice immunized with BCG-MSP1C, the parent BCG, LPS, or PBS-T80, in the presence or absence of CRX 527, were measured in order to assess the influence of the TLR4 agonist on IL-1β production. Overall, the expression of IL-1β, which was analysed from the cultured supernatants using ELISA, was found to be greater in all groups with the presence of CRX 527 (Figure 2). The highest significant increase of IL-1β in the presence of CRX 527 was detected in the BCG-MSP1C-immunised groups derived from the spleen (60.16 ± 0.53 pg/ml) (P = 0.000), but a non-significant trend was detected in the liver and lymph node. Secondly, a significant increase was detected in the BCG + CRX 527-immunised groups derived from the liver (58.77 ± 0.60 pg/ml) (P = 0.008), followed by the spleen (57.82 ± 0.44 pg/ml) (P = 0.000), but a non-significant trend was detected in the lymph node. A significant increase was also detected in the LPS + CRX-527-immunised group, derived from the liver (58.06 ± 0.25 pg/ml) (P = 0.000). A non-significant trend was detected in the PBS-T80 + CRX 527-immunised group.

Expression of IL-1β in lymphocytes-derived tissue. (a) Liver, (b) Spleen and (c) Lymph node of mice immunised with PBS-T80, LPS-T80, BCG or BCG-MSP1C in the absence or presence of TLR4 agonist, CRX 527. The concentration of TNF-α were presented in mean ± SEM. Sample size, n = 6 per group with 95% confidence interval.
Measurement of IFN-γ Productions
IFN-γ levels in lymphocyte-derived tissues from mice immunized with BCG-MSP1C, the parent BCG, LPS, or PBS-T80, in the presence or absence of CRX 527, were measured in order to assess the influence of the TLR4 agonist on IFN-γ production. Overall, the expression of IFN-γ, which was analysed from the cultured supernatants using ELISA, was also found to be greater in all groups with the presence of CRX 527 (Figure 3). Different patterns of IFN-γ production were observed in the results. The highest significant increase of IFN-γ in the presence of CRX 527 was detected in the BCG-immunised groups derived from the liver (216.18 ± 1.66 pg/ml) (P = 0.003), followed by the spleen (211.93 ± 0.52 pg/ml) (P = 0.000) and the lymph node (202.78 ± 0.04 pg/ml) (P = 0.000). Secondly, a significant increase was detected in the BCG-MSP1C + CRX 527-immunised groups derived from the liver (207.98 ± 0.82 pg/ml) (P = 0.000), followed by the spleen (195.87 ± 0.30 pg/ml) (P = 0.000) and the lymph node (178.54 ± 0.05 pg/ml) (P = 0.000). A significant increase of IFN-γ was also detectable in the LPS + CRX 527-immunised groups, derived from the spleen (193.17 ± 0.76 pg/ml) (P=0.000), followed by the liver (185.75 ± 0.89 pg/ml) (P = 0.000) and the lymph node (174.53 ± 0.17 pg/ml) (P = 0.000). In the PBS-T80 + CRX 527-immunised group, a significant increase of IFN-γ was also detectable in the liver (180.00 ± 0.64 pg/ml) (P = 0.001), followed by the lymph node (180.25 ± 0.13 pg/ml) (P = 0.000) and the spleen (177.86 ± 0.17 pg/ml) (P = 0.000).

Expression of IFN-γ in lymphocytes-derived tissue. (a) Liver, (b) Spleen and (c) Lymph node of mice immunised with PBS-T80, LPS-T80, BCG or BCG-MSP1C in the absence or presence of TLR4 agonist, CRX 527. The concentration of TNF-α were presented in mean ± SEM. Sample size, n = 6 per group with 95% confidence interval.
Measurement of IL-4 Productions
IL-4 levels in lymphocyte-derived tissues from mice immunized with BCG-MSP1C, the parent BCG, LPS, or PBS-T80, in the presence or absence of CRX 527, were measured in order to assess the influence of the TLR4 agonist on IL-4 production. Overall, the expression of IL-4, which was analysed from the cultured supernatants using ELISA, was also found to be greater in all groups with the presence of CRX 527 (Figure 4). The highest significant increase of IL-4 in the presence of CRX 527 was detected in the BCG-MSP1C-immunised groups derived from the spleen (20.49 ± 0.15 pg/ml) (P = 0.000), followed by the liver (20.49 ± 0.08 pg/ml) (P = 0.000) and the lymph node (17.91 ± 0.04 pg/ml) (P = 0.013). Secondly, a significant increase was detected in the BCG + CRX 527-immunised groups derived from the spleen (18.87 ± 0.26 pg/ml) (P = 0.000), followed by the liver (18.01 ± 0.03 pg/ml) (P = 0.000), but a non-significant trend was observed in the lymph node. A non-significant trend was also detected in the LPS + CRX 527- and PBS-T80 + CRX 527-immunised groups.

Expression of IL-4 in lymphocytes-derived tissue. (a) Liver, (b) Spleen and (c) Lymph node of mice immunised with PBS-T80, LPS-T80, BCG or BCG-MSP1C in the absence or presence of TLR4 agonist, CRX 527. The concentration of TNF-α were presented in mean ± SEM. Sample size, n = 6 per group with 95% confidence interval.
Discussion
This study aims to investigate the possible role of a TLR4 agonist in the production of several intracellular and inflammatory cytokines—TNF-α, IL-1β, IFN-γ, and IL-4. The TLR4 agonist promotes the upregulation of costimulatory molecules and secretion of intracellular and inflammatory cytokines15. The results obtained showed that lymphocyte-derived tissues of mice immunised with PBS-T80, LPS-T80, BCG, or BCG-MSP1C in the presence of the TLR4 agonist CRX-527 produced higher levels of the investigated intracellular and inflammatory cytokines in all examined organs compared to the groups in the absence of CRX-527. The highest secreted cytokine protein was IFN-γ (Figure 3), which was produced at levels of 150.0 to 250.0 pg/ml. Meanwhile, TNF-α and IL-1β were secreted at relatively low levels (Figure 1, Figure 2), ranging between 50.0 and 97.0 pg/ml. However, IL-4 production was the most difficult to induce, resulting in a mean concentration of only 15–20 pg/ml. For all four cytokines, the optimal stimulus came from the group of mice treated with BCG and BCG-MSP1C with CRX-527. Observations showed that when the TLR4 agonist was present, the expression of all cytokines was also elevated. This finding aligns with other cases of malaria infection that showed a similar pattern of elevated inflammatory cytokine release stimulated by other TLR4 agonists16, 17. Earlier research also showed an increased production of serum immunoglobulins by stimulation with the TLR4 agonist lipopolysaccharide (LPS)18. This hypothesis was in agreement with a previous study by Abbas and Suppian, where they used a TLR4 inhibitor (TAK-242) to define the role of TLR4 in the production of serum immunoglobulins and cytokines (IFN-γ and IL-4)6. When the TLR4 inhibitor was absent, the expression of all cytokines was elevated. This result showed the correlation between TLR4 agonists and inhibitors in activating Toll-like Receptors (TLRs). According to previous studies, TLR4-mediated responses exhibit substantial individual and evolutionary variability19, 20. Furthermore, several bacterial diseases have been linked to variations in LPS structures and TLR4's differential recognition of these structures21. Variations in this reaction pattern are possible and could be a factor in disease onset.
A study by Clark. examined how BCG protects mice against hemoprotozoa, becoming a stepping stone toward discovering a connection between inflammatory cytokines and malaria illness development22. Inflammatory cytokines such as TNF-α and IL-1β have been found to play central roles in many infectious diseases, including Buruli ulcer, tuberculosis, malaria, and others23. The fact that these cytokines function together in tandem is widely documented24, 25. Proinflammatory cytokines are crucial in initiating and amplifying the immune response against infectious pathogens since they are involved in recruiting immune cells to the site of infection, promoting inflammation, and facilitating pathogen clearance26. TNF-α can trigger host defence mechanisms, both innate and adaptive27, while Interleukin-1 (IL-1) stimulates the production of other proinflammatory cytokines and promotes the recruitment of immune cells to inflammation sites28. There is an increase in the production of the proinflammatory cytokines TNF-α, IL-6, and IL-1β in studies using PBMCs of -infected patients with severe malaria29. Similarly, our results also showed a significant increase in TNF-α and IL-1β production in the lymphocyte-derived tissues of mice treated with BCG and BCG-MSP1C in response to stimulation by the TLR4 agonist CRX-527.
Meanwhile, IL-1β, which was first discovered as an endogenous pyrogen, is a potent proinflammatory cytokine typically activated in macrophages. It exerts a stimulatory effect on Cluster of Differentiation 4 (CD4) T cells and promotes their differentiation into T helper cell lineages30. Additionally, proinflammatory cytokines of the IL-1 family, particularly IL-1β and IL-18, play crucial roles in antimicrobial host defence31. IL-1β is usually triggered in macrophages following inflammasome sensing of infection or pathogen invasion, resulting in caspase-1 processing of IL-1β and subsequent release32. The finding that IL-1β is associated with parasitic clearance has drawn attention to understanding distinct innate and adaptive immune responses in human diseases.
A previous study by Abbas and Suppian also investigated the role of TLR4 in the production of IFN-γ and IL-4 by splenocytes of mice immunised with PBS-T80, BCG, or rBCG in the absence or presence of a TLR4 inhibitor, where the results showed a significant increase in the production of both cytokines in the sera of mice immunised with BCG-MSP1C compared to the control6. Meanwhile, this study investigated the role of TLR4 in the production of IFN-γ and IL-4 in lymphocyte-derived tissues (liver, spleen, and lymph node) of mice immunised with PBS-T80, LPS, BCG, and BCG-MSP1C in the absence or presence of a TLR4 agonist. The results also showed a significant increase in the production of both cytokines in the BCG and BCG-MSP1C immunised groups with CRX-527 compared to the control. Our findings are supported by another study that found IFN-γ mediates parasite clearance, which is necessary for a protective immune response against visceral leishmaniasis33. IFN-γ, released by Type 1 T helper cells (Th1), Cluster of Differentiation 8 (CD8), and CD4CD8- T lymphocytes, activates macrophages of the M1 phenotype (proinflammatory) by stimulating TLR receptors, which subsequently release nitrogen and oxygen radicals (nitric oxide, NO)34. These molecules inhibit parasite growth and degenerate malarial parasites by exerting oxidative stress35.
This study also analysed the role of the TLR4 agonist in the production of the anti-inflammatory cytokine IL-4, given IL-4’s known role in controlling inflammation through the repression of proinflammatory cytokine production. Consequently, IL-4 was found to be the most difficult to induce, with a mean concentration of only 15–20 pg/ml, and was most abundant in BCG-MSP1C-immunised mice. The high variability in IL-4 levels (15–20 pg/ml) may suggest biological heterogeneity in the population, meaning that genetic, environmental, and physiological factors could affect this finding. This is similar to observations in children with severe malaria anemia in Franceville, southeast Gabon, where only a small percentage of IL-4 was detected in their blood samples36. Previous studies found that during malaria, the frequency of IL-4-producing CD4 T cells decreased, while the frequency of other CD4 T cells capable of helping B cells produce -specific antibodies increased37. The Th1 cytokine response was downregulated by Type 2 T helper cells (Th2) (anti-inflammatory) cytokines, which promoted B cell activation and the production of -specific antibodies38. Anti-inflammatory Th2 cytokines, including IL-4 and Interleukin-13 (IL-13), regulate the humoral immune response by inhibiting Th1 cytokine production and concurrently contributing to parasite clearance39, 40. According to several studies, the simultaneous release of proinflammatory cytokines such as IL-1β, TNF-α, and the anti-inflammatory cytokine Interleukin-10 (IL-10) is required to activate the innate immune response to clear parasitic invasion41, 42. Since our results showed a simultaneous reaction in the secretion of the intracellular and inflammatory cytokines TNF-α, IL-1β, IFN-γ, and IL-4 in lymphocyte-derived tissues of mice immunised with BCG-MSP1C in the presence of CRX-527, we suggest the possible use of TLR4 as an adjuvant to enhance the immune response of a malaria vaccine candidate.
These findings also suggest that the TLR4 agonist CRX-527 is suitable for use as an adjuvant compared to other TLR4 agonists, such as lipopolysaccharide (LPS) or monophosphoryl lipid A (MPLA), because it offers unique advantages in adjuvant efficacy and safety. CRX-527 was designed to stimulate immune responses and induce inflammation and fever while minimising toxicity43.
Conclusion
In conclusion, this study highlighted the role of the TLR4 agonist (CRX-527) in stimulating the expression of intracellular and inflammatory cytokines in lymphocyte-derived tissues of mice immunised with BCG-MSP1C. Our results showed a significant increase in the expressions of all the investigated inflammatory cytokines, with the highest amounts derived from the spleen, followed by the lymph node and liver. These findings support the hypothesis that a correlation between proinflammatory and anti-inflammatory cytokines, stimulated by TLR4, is necessary to activate potent immune responses. However, to confirm the findings, further studies should be conducted to elucidate the adaptive immune response against rBCG expressing the MSP-1C of in lymphocyte-derived tissue and to detect the role of a TLR4 agonist in activating the adaptive immune response for a malaria vaccine candidate. An in vivo study may also be conducted to further clarify the protective efficacy of rBCG expressing the MSP-1C of as a potential malaria vaccine candidate.
Abbreviations
ACK Lysis- ammonium–chloride–potassium Lysis buffer, ANOVA- one-way analysis of variance, ARASC- Animal Research and Service Centre, Avidin HRP- Avidin horseradish peroxidase, BCG- Bacillus Calmette–Guérin (BCG), BCG-MSP1C- Mycobacterium bovis of Bacillus Calmette-Guerin expressing C-terminus merozoite surface protein-1 of Plasmodium falciparum, CD4- Cluster of Differentiation 4, CD-8- Cluster of Differentiation 8 CFU- Colony Forming Unit, CFU/mL- Colony Forming Unit per millilitre, CO2 incubator- Carbon dioxide Incubator, ELISA- Sandwich Enzyme-Linked Immunosorbent Assay, FBS- Fetal Bovine Serum, IFN-γ- interferon-gamma, IL-1β- Interleukin-1 beta, IL-4- Interleukin-4, IL-10- Interleukin-10, IL-13- Interleukin-13, LPS- lipopolysaccharide, mg/kg- Milligram per kilogram, ml- Millilitre, µg/ml- Micrograms per millilitre, µl- Microliter, MPLA- monophosphoryl lipid A, NO- nitric oxide, OADC- oleic acid, albumin, dextrose, and catalase, OD- optical density, PBS- Phosphate Buffered Saline, pg/ml- picograms per millilitre, rBCG- recombinant BCG, RPMI 1640 medium- Roswell Park Memorial Institute 1640 medium, SEM- Standard error of mean, SPSS- Statistical Package for the Social Sciences, Th1- Type 1 T helper cells, Th2- Type 2 T helper cells, TLR4- Toll-like receptors 4, TLRs- Toll-Like Receptor, TNF-α- tumor necrosis factor-alpha, USM- Universiti Sains Malaysia
Acknowledgments
We thank all the staffs from Animal Research and Service Centre, Universiti Sains Malaysia, Kelantan, Malaysia, for their help rendered during conduction of animal experiments.
Author’s contributions
Norlaily Hanifi: Designed and conducted the experiment, formal analysis and writing the manuscript. Munirah Zakaria: Conceptualisation, data curation, formal analysis, writing – review and editing. Rapeah Suppian: Validation, supervision, writing – review and editing. All authors read and approved the final manuscript.
Funding
This research was supported by the Ministry of Higher Education, Fundamental Research Grant Scheme (Grant number: FRGS/ 203/PPSK/6171298).
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the animal care guidelines and approval from the Universiti Sains Malaysia (USM) Animal Ethics Committee, (USM/IACUC/2020/ (125) (1099)).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

