Integrative Analysis of an Ayurvedic Polyherbal Decoction:From Phytochemistry to Pharmacological Efficacy
- Sri Ramachandra Faculty of Pharmacy, Sri Ramachandra Institute of Higher Education and Research, Chennai - 600116, Tamil Nadu, India
- School of Life Sciences, B. S. Abdur Rahman Crescent Institute of Science and Technology, Chennai- 600 048, Tamil Nadu, India
- Department of Anatomy, Sree Balaji Medical College and Hospital, Chennai – 600044, Tamil Nadu, India
- AURA Biotechnologies Private Limited, Chennai - 600095, Tamil Nadu, India
- Department of Bioinformatics, Vels Institute of Science Technology and Advanced Studies, Pallavaram, Chennai 600117, Tamil Nadu, India
- Sri Ramachandra Faculty of Clinical Research, Sri Ramachandra Institute for Higher Education and Research, Chennai - 600116, Tamil Nadu, India
- B Aatral Biosciences Private Limited, Bangalore, 560091, Karnataka, India
Abstract
Background: Ayurvedic medicine offers a wealth of traditional formulations, yet many remain scientifically underexplored. One such formulation, a polyherbal decoction composed of Drynaria quercifolia, Eclipta prostrata, Phyllanthus amarus Schumach, Phyllanthus emblica, Piper longum, Piper nigrum, Terminalia bellirica, Terminalia chebula, and Zingiber officinale Roscoe, is traditionally known for its anti-inflammatory, antioxidant, and gastroprotective properties. Despite its widespread use, limited scientific data exist regarding its phytochemical constituents and pharmacological mechanisms. To investigate the chemical composition and therapeutic potential of the Ayurvedic polyherbal decoction using modern analytical and biological techniques, aiming to bridge traditional knowledge with contemporary scientific validation.
Methods: Phytochemical profiling was performed using High-Performance Thin Layer Chromatography (HPTLC) and Gas Chromatography-Mass Spectrometry (GC-MS) to identify bioactive constituents. Pharmacological evaluations included antioxidant (DPPH and ABTS assays), anti-inflammatory (nitric oxide inhibition), and cytotoxicity (MTT assay) analyses. Additionally, molecular docking and molecular dynamics simulations were employed to assess ligand-protein interactions. DNA binding and nitric oxide suppression assays further substantiated the therapeutic relevance of this compound.
Results: The decoction exhibited significant antioxidant and anti-inflammatory activity with minimal cytotoxicity. GC-MS and HPTLC analyses revealed the presence of multiple bioactive compounds with known pharmacological properties. Molecular docking and dynamics simulations confirmed strong binding affinities between decoction constituents and inflammation-related protein targets, supporting potential mechanisms of action. DNA binding studies and nitric oxide suppression confirmed the formulation’s efficacy in modulating oxidative stress and inflammatory responses.
Conclusion: This study demonstrates the therapeutic potential of the Ayurvedic polyherbal decoction through comprehensive phytochemical and pharmacological analyses. The integration of traditional knowledge with modern scientific tools underscores its relevance in contemporary healthcare, providing a natural and effective alternative to synthetic pharmaceuticals for managing inflammation, oxidative stress, and gastrointestinal disorders.
Introduction
Natural healing methods have long been integral to global therapeutic practices1, 2. Polyherbal extracts are widely used in South India to treat inflammatory conditions, oxidative stress, and gastrointestinal issues3, 4. One such polyherbal decoction is composed of nine herbal plants, including and . The enumerated phytochemicals in the polyherbal extract include numerous bioactive compounds believed to confer therapeutic properties5. Moreover, it establishes a standardized approach for extraction and processing that utilizes decoction to concentrate the active compounds. This liquid is suitable for oral administration. A further benefit of traditional Ayurvedic medicines is the adaptability of the result6. This therapeutic approach typically yields an internally administered solution, but a tablet formulation is also feasible7. Nonetheless, applying these formulations is prevalent, and comprehension of the evidence supporting these interventions and the specific active components that underpin them needs to be improved, necessitating interdisciplinary collaboration to enhance our understanding of their therapeutic efficacy8.
The polyherbal decoction contains distinct phytochemical components. These compounds include alkaloids, flavonoids, tannins, glycosides, and amino acids that affect their anti-inflammatory, antioxidant, and gastroprotective properties9. Traditionally, polyherbal decoctions are recognized for enhancing the body's immune system, alleviating inflammation, and promoting overall wellness10. Nonetheless, empirical information substantiating these compounds' chemical composition, pharmacological properties, and prospective therapeutic applications is limited11. This deficiency in comprehension presents an opportunity to investigate and validate its efficacy in treating this condition using modern scientific methodologies12.
These investigations are being augmented by High-Performance Thin-Layer Chromatography (HPTLC) and Gas Chromatography–Mass Spectrometry (GC-MS), providing a robust foundation for the phytochemical examination of traditional treatments13, 14. These methodologies facilitate the identification of molecules with certain activities or attributes, enhancing the correlation between chemical structure and biological action. Furthermore, the comprehensive bioassay capabilities, including antioxidant, anti-inflammatory, and cytotoxicity assessments, provide insights into the formulation's pharmacology15. Integrating molecular docking and molecular dynamics simulations enhances comprehension of the binding affinities of essential bioactive chemicals and their target proteins, perhaps elucidating their pharmacological mechanisms16, 17.
The current study aims to provide a scientific foundation for the traditional applications of polyherbal decoction by analyzing its chemical composition and pharmacological effects. This study aims to demonstrate the efficacy of the formulated compounds through phytochemical analysis, HPTLC, nitric oxide inhibition, DNA binding, and molecular docking studies while exploring their potential applications in anti-inflammatory, antioxidant, and anticancer activities. The research seeks to elucidate the mechanism of the formulation by identifying molecular targets, including interleukin-8 (IL8), to discover novel treatments for inflammatory and gastrointestinal disorders18, 19.
This study further aims to integrate traditional herbal medical practices with contemporary scientific research to guarantee the use of safe and effective herbs in medicine. The planned extensive investigation would enhance the practical use of polyherbal decoction and further the identification of associated bioactive substances with potential therapeutic benefits. This aligns with the contemporary global trend in which several cultures incorporate traditional medicine into their healthcare systems, advocating for the use of natural products over synthetic pharmaceuticals.
Methods
Decoction preparation
The nine plants have been collected and authenticated. Using the cold percolated method, aqueous extract of all plants (, and ) has been prepared. All plants are collected, identified, and authenticated by Dr. K. Jeyaprakash, Botanist, Institute of Natural Science Research (Registered under the Indian Trusts Act), Devadanapatti - 625602, Tamil Nadu, India. Equal amounts of plant powder were weighed and soaked in distilled water for 3 days from time to time. After filtration, the extracts were concentrated and dehydrated to yield a semi-solid extract, which was stored at 4°C for further studies.
Preliminary Phytochemical Analysis
The polyherbal decoction was prepared using standard protocols. A dried semi-solid substance will be weighed to 100 mg and dissolved in 25 ml of distilled water for initial phytochemical screening10, 20. This analysis aims to identify the presence of several phytoconstituents, including alkaloids, quinones, polysaccharides, and terpenoids. Furthermore, the formulation will be tested to check for triterpenoids, glycosides, phytosterols, phenolics, bound compounds, steroids, flavonoids, amino acids, and tannins. These phytoconstituents will be identified using conventional methods specific to each group of compounds.
High-Performance Thin Layer Chromatography (HPTLC)
The chromatographic instrumentation utilized in this study was a CAMAG® Automatic TLC Sampler 4 (ATS 4). The plate development process was conducted in a glass chamber designed explicitly for chromatography13. The plate was evaluated using a TLC scanner 3, in conjunction with winCATS 4 software version 1. The samples were subsequently analyzed using High-Performance Thin Layer Chromatography (HPTLC) on aluminum-backed plates (10 x 10 cm) that were pre-coated with Merck silica gel 60 F254 (0.2 mm thickness). The plates were cleaned using methanol, heated to 60°C for five minutes, and subsequently, the sample was applied in four distinct quantities of ethyl acetate extract, namely 2 µL, 4 µL, 8 µL, and 12 µL, respectively. The samples were used as bands with a width of 8 mm at various positions on the plates, precisely 11 mm from the bottom and 15 mm from the side edges, using a Camag Linomat 5 semi-automatic applicator. The bands were desiccated by concurrently passing a nitrogen stream. The plate development was conducted using the ascending mode in a twin-trough glass chamber measuring 10 × 10 cm. The mobile phase consisted of n-hexane, Ethyl acetate, methanol, and formic acid in the 60:40:2.5:2.5 (v/v/v/v) ratio. The plates were subjected to densitometric scanning using a Camag TLC scanner three equipped with winCATS software, version 1.4.8, under UV light at a wavelength of 254 nm. The scanning process was conducted using a slit width of 5 mm by 0.45 mm, with a velocity of 20 mm/s and a data resolution of 0.121. The Rf values of the sample were measured, and photographs were captured using visible light and UV light at 254 and 366 nm wavelengths in the CAMAG REPROSTAR 3 photo documentation chamber. The peak counts, heights, peak areas, peak displays, and densitograms were acquired and examined using winCATS software.
Gas Chromatography/Mass Spectrometry (GC/MS)
A fraction of the identified polyherbal decoction was purchased from a renowned Ayurvedic store in Chennai. The sample was analyzed using Gas Chromatography-Mass Spectrometry (GC-MS) as per established protocols Gas Chromatography-Mass Spectrometry (GC–MS) BT - Encyclopedia of Geochemistry14. The study utilized an Agilent GC-MS system consisting of a G3440A gas chromatograph and a 7890A mass spectrometer, namely the 7000 Triple Quad GCMS model, equipped with a mass spectrometry detector. 100 µL of polyherbal decoction was combined with 1 mL of an ethanol solvent using a vortex stirrer for 10 seconds to prepare the sample. The transparent extract was obtained using this procedure, and the sample was then processed for gas chromatography-mass spectrometry (GC-MS) analysis. The GC-MS analysis was conducted using a DB-5MS column with dimensions of 30 mm × 0.25 mm ID × 0.25 µm. The column consists of 5% phenyl and 95% methyl polysiloxane. The study used electron impact mode at an energy level of 70 electron volts (eV). Helium, with a purity of 99.999%, was used as the carrier gas at a flow rate of 1 mL/min. The injector, auxiliary, and ion source temperature settings were 280°C, 290°C, and 280°C, respectively. The oven temperature is programmed to start at 50°C and remain constant for 1.0 minutes. Then, the temperature increases at a rate of 40°C per minute until it reaches 170°C, which remains constant for 4.0 minutes. Finally, the temperature is increased at a rate of 10°C per minute until it reaches 310°C, which remains constant for 10 minutes. A mass range of 45 to 450 Da was surveyed, and the entire duration of the run was 32.02 minutes. The compounds were characterized by comparing them to the reference spectra found in the NIST and WILEY GC-MS libraries22.
ABTS (2,2'-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)) radical scavenging assay The ABTS radical cation decolorization test measured the antioxidant activity23. ABTS radical cations were produced by combining equal volumes of a 7 mmol/L ABTS solution with a 2 mmol/L solution of sodium metabisulfite (NaSO) and potassium persulfate (KSO) at a concentration of 45 mmol/L. Subsequently, the mixture was placed in a dark environment at room temperature for 12 hours before utilization. The ABTS solution that was obtained was subsequently diluted with ethanol. The test samples were generated at concentrations ranging from 5 to 320 μg/mL, and the standard, ascorbic acid, was also synthesized at concentrations ranging from 5 to 320 μg/mL via serial dilution. The reaction solution is combined with 2 ml of the diluted ABTS solution. Once the reaction mixtures were created, they were allowed to sit at room temperature for 6 minutes before measuring the absorbance levels of the solutions using an ultraviolet-visible spectrophotometer at a wavelength of 734 nm. The experiment was conducted three times. The percentage of the radical scavenging effect of the samples was assessed using the formula employed to measure the ABTS radical scavenging activity. ABTS radical scavenging effect (%) = [(A - A)/A] ×100 Where A is the absorbance of control; A is the absorbance of test.
Ferric Reducing Antioxidant Potential Assay
The spectrophotometric method of Benzie & Strain, 199924 was used to assess the antioxidant activity of polyherbal decoction. This activity involves the conversion of Fe³⁺-TPTZ (colorless) to Fe²⁺-tripyridyltriazine (blue-colored complex) by donating an electron at low pH. The reaction was monitored using a spectrophotometer set at a wavelength of 593 nm. The Ferric Reducing Antioxidant Power (FRAP) reagent was prepared by combining 300 mM acetate buffer, TPTZ (10 mM) in 40 mM HCl, and 20 mM FeCl₃·6H₂O in a 10:1:1 ratio. The mixture was incubated at 37°C. A freshly manufactured FRAP reagent (3.995 mL) was prepared by combining it with the test samples and standard ascorbic acid at varying concentrations (5-320 μg/mL). The mixtures were incubated for 30 minutes at 37°C. During this time, the Fe³⁺-TPTZ complex underwent reduction, resulting in the creation of Fe²⁺ and the appearance of vivid blue color. The absorbance of this compound was determined at a wavelength of 593 nm using a reagent blank. The reagent blank was made by combining 3.995 mL of FRAP reagent with 5 μL of distilled water. The assessments were conducted thrice.
Protein Denaturation Assay
The anti-inflammatory efficacy of polyherbal decoction was assessed using a modified version of the protein denaturation method25. The diclofenac sodium medication was prepared using DMSO and diluted with a phosphate buffer (0.2 M, pH 7.4). The final concentration of DMSO in all solutions was kept below 2.5%. The drug was diluted in solutions of 4 mL with concentrations of 5, 10, 20, 40, 80, 160, and 320 μg/mL. These diluted solutions were then added to 1 mL of a 1 mM albumin solution in phosphate buffer. The mixture was left undisturbed for 15 minutes at a temperature of 37°C. The denaturation process was done by subjecting the reaction mixture to heat at 60°C for 15 minutes in a water bath. The turbidity of the samples was measured at a wavelength of 660 nm after they were cooled. The same process was also employed for the standard medication, and its level of cloudiness was recorded. The percentage inhibition of protein denaturation was calculated by comparing it to a control solution that did not contain the medication25.
Membrane Stabilisation Assay
Five milliliters of fresh whole human blood were drawn from healthy adult volunteers following informed consent, placed into a heparinized tube, and aliquoted into centrifuge tubes. The blood was spun at 3000 rpm for 10 minutes, and the pellets were washed thrice with normal saline at the same volume. The study protocol was approved by the Institutional Review Board (or Ethics Committee) of Bharath Institute of Higher Education and Research, Chennai (Protocol code BIHER/BSCI/IHEC/2021/15 dated 27.11.2021). The volume of the blood was then measured, and the blood was diluted to a 40% v/v suspension in an isotonic solution (10 mM sodium phosphate buffer)26. For the assay, a 40% RBC suspension was prepared by mixing 1 mL of packed RBCs with 1.5 mL of normal saline. Then, 1 mL of the suspension was incubated with varying concentrations of the polyherbal decoction, ranging from 50 to 1600 μg/mL, and the standard drug Diclofenac sodium. The control consisted of 1 mL of RBC suspension mixed with 1 mL of isotonic saline without the addition of any test substances. The reaction mixtures were incubated in a water bath at 56°C for 30 minutes. After incubation, the tubes were allowed to cool to room temperature and then centrifuged at 2500 rpm for 5 minutes. The absorbance of the supernatant was then read at 560 nm. Percent membrane stabilization activity was determined using the following equation: % Inhibition of Haemolysis = (OD of control - OD of test)/OD of control × 100.
Cell Culture and Cytotoxicity Assay
The antitumor properties of polyherbal decoction were assessed for cytotoxicity using an MTT assay on the A549 human lung carcinoma cell line. A549 cells were sourced from NCCS Pune, India, and maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS; Genetix Biotech, India), penicillin/streptomycin (Sigma) at 37°C in a humidified atmosphere containing 5% CO. Human cells were plated at a density of 4,000 cells per well in 96-well plates and allowed to grow for 24 hours27. Polyherbal decoctions were dissolved in phosphate-buffered saline (PBS) to prepare 10 mg/mL stock solutions, and the final concentrations ranged from 1 to 1000 µg/mL. The treated cells were incubated at 37°C for 48 hours, and then 50 µL of 5 mg/mL MTT solution was added to each well and further incubated for 3 hours. Subsequently, the medium was removed from the wells, and the formed formazan crystals were dissolved in 100 µL DMSO. The absorbance was then taken at 570 nm using a Synergy HT microplate reader. Percentages of cell survival were also computed, and dose-response curves were plotted to obtain GI values. Microscopic examination of cell morphology was done at the end of 48 hours of treatment, and the pictures were taken to compare morphological alterations.
Statistical Analysis
Data are consistently presented as the average ± the standard error of the mean (SEM), allowing readers to assess the precision of the average. A one-way analysis of variance (ANOVA) was employed to determine if significant differences existed among the groups under various experimental settings. Dunnett’s multiple-comparison test was used after ANOVA to provide specific comparisons between each experimental group and the control group, reducing the likelihood of Type I errors by appropriate adjustment for multiple testing. Calculating 95% confidence intervals enables the estimation of the range within which the actual population parameters are likely to lie. Selecting a P-value threshold of < 0.05 assured that the observed mean differences were likely not due to chance. Arriving at these conclusions through a diverse array of evidence enhances their credibility and comprehensibility. All experimental assays were performed in triplicate (n = 3) to ensure reproducibility and statistical reliability. The sample sizes were selected based on preliminary studies and established protocols in similar in vitro experimental models. This approach strikes a balance between practical feasibility and the need for robust statistical analysis. Results are reported as mean ± standard error of the mean (SEM), and all assays were repeated independently to confirm consistency.
Molecular Docking
The extract's chemical compounds identified through GCMS analysis were evaluated using molecular docking techniques to determine their interaction with the Interleukin 8 protein target associated with irritable bowel syndrome28. The crystal structure of the target protein (PDB: 3IL8) indicates its role in the host defense system as a chemotactic factor29. Autodock Vina was chosen for the molecular docking process to model the protein-ligand complex30. The assessment of receptor-ligand binding affinities was organized and presented in a table to identify active compounds.
Molecular Dynamics Simulations
A molecular dynamics investigation was conducted on a protein-phytocompound combination. Molecular identification was performed by capturing frames throughout the trajectory creation using the GROMACS 2019 program31. Topologies for small molecules were generated with the PRODRG2 server, while the SPC water model and GROMOS96 43a1 force field were employed to define the protein topologies32. The cubic box enclosure facilitated solvation and energy minimization for the constructed complex system. To neutralize the solution, sodium (Na) and chloride ions were introduced to the receptor-ligand complex system. Energy minimization was performed utilizing the steepest descent integrator. The temperature and pressure were maintained at 300 K and 1 atm, respectively, with a leap-frog integrator. All molecular dynamics simulations were conducted in NVT and NPT ensembles, with save frame intervals exceeding 50 ns.
Phytochemical analysis of polyherbal decoction showing the presence (+) or absence (-) of various bioactive compounds
|
S.No |
Test |
Polyherbal decoction |
|---|---|---|
|
1 |
Alkaloids |
+ |
|
2 |
Quinones |
- |
|
3 |
Carbohydrate |
+ |
|
4 |
Terpenoids |
- |
|
5 |
Triterpenoids |
- |
|
6 |
Glycosides |
+ |
|
7 |
Phytosterols |
+ |
|
8 |
Phenolic compounds |
- |
|
9 |
Steroids |
- |
|
10 |
Flavonoids |
+ |
|
11 |
Amino acids |
+ |
|
12 |
Tannins |
+ |
Results
Preliminary Phytochemical Screening
The phytochemical analysis of polyherbal decoction revealed the presence of multiple bioactive chemicals that may contribute to the drug's therapeutic effects. Based on the positive results obtained in the corresponding tests, the formulation contained alkaloids, carbohydrates, glycosides, phytosterols, flavonoids, amino acids, and tannins (
Phytosterols, which are linked to cholesterol regulation, play a role in enhancing heart health. The flavonoids in the polyherbal decoction have been recognized for their antioxidant, anti-inflammatory, and antiviral characteristics36. Proteins, composed of amino acids, may play a role in tissue regeneration and metabolic processes37. The plant also contains tannins, which have been found to possess astringent, antibacterial, and anti-inflammatory properties38. However, examining quinones, terpenoids, triterpenoids, phenolic compounds, and steroids reveals that these compounds do not play a role in bioactivity. The results unequivocally support the traditional use of polyherbal decoctions for medicinal purposes and contribute to our understanding of their potential pharmacological effects. Further research is needed to thoroughly clarify the pharmacological effects and therapeutic efficacy of these substances in humans.

HPTLC analysis of polyherbal decoction. (A) Chromatographic separation of phytochemicals visualized at 254 nm (left) and 366 nm (right) showing distinct bands. (B) 3D densitometric profile of tracks scanned at 254 nm.
HPTLC Profile
The High-Performance Thin-Layer Chromatography (HPTLC) analysis revealed twelve distinct peaks in the sample when exposed to ultra-violet (UV) light at 254 nm and 366 nm39, as shown in Figure 1. This indicates that the sample hosts numerous compounds. Two prominent peaks were found, indicating the presence of two main chemicals in the sample. Hence, our findings demonstrate the diverse range of phytochemicals present in the sample and highlight the need for further investigation to identify the bioactive molecules. The presence of a peak indicated no discernible distinction between the two samples. The data suggests that a 6% portion of the overall curve area could include Compound A despite its minor component. However, this small fraction could still hold significant importance. Simultaneously, a prominent peak at an Rf value of 0.68 corresponds to a representation of 44. The results obtained a reliability of 48% for the entire area, suggesting that Compound B is the primary component of the sample. The significant abundance of Compound B implies that it likely accounted for the majority, if not the entirety, of the biological or therapeutic impact observed in the sample. The discovery of these compounds could be crucial in establishing the sample's efficacy and potential application in a more robust formulation.

A GC-MS chromatogram of the polyherbal decoction shows total ion current (TIC) across retention times. Prominent peaks are observed at 5.726 min, 9.707 min, 16.090 min, 20.378 min, 24.211 min, 25.764 min, and 28.113 min, indicating the presence of major bioactive compounds in the sample. The data suggests a complex mixture of volatile components consistent with the diverse phytochemical profile of the traditional Ayurvedic formulation.
It presents the retention values, types of possible compounds, their molecular formulae, molecular mass, peak area, and the medicinal roles of each compound, as shown in the GC MS profile of polyherbal decoction
|
Retention time |
Molecules |
Mol. formula |
Mo. Wt. |
% peak area |
Possible medicinal role |
|---|---|---|---|---|---|
|
5.68 |
Benzoic acid |
C7H6O2 |
122 |
37.91 |
Acidifier, Arachidonic acid Inhibitor, Increase Aromatic Amino acid decarboxylase activity, Inhibit production of uric acid, Urine acidifier |
|
15.73 |
11-Octadecenoic acid, methyl ester |
C19H36O2 |
296.3 |
0.92 |
Arachidonic acid Inhibitor, Increases Aromatic Amino acid decarboxylase activity, Inhibits production of uric acid, Urine acidifier, Catechol O Methyl transferase inhibitor, Methyl donor, Methyl guanidine inhibitor, Acidifier |
|
16.09 |
17-Octadecynoic acid |
C18H32O2 |
280.2 |
0.59 |
Arachidonic acid Inhibitor, Increases Aromatic Amino acid decarboxylase activity, Inhibit production of uric acid, Urine acidifier, Catechol O Methyl transferase inhibitor, Methyl donor, Methyl guanidine inhibitor, Acidifier |
|
17.27 |
2-Cyclohexyl-2,5-cyclohexadiene-1,4-dione, 4-oxime |
C12H15NO2 |
205.1 |
1.11 |
Not known |
|
18.03 |
Gingerol |
C17H26O4 |
294.2 |
0.87 |
anti-inflammatory, anti-angiogenic, antibacterial and antifungal |
|
20.38 |
1-Heptatriacotanol |
C37H76O |
536.6 |
15.84 |
Antibacterial, anticancer, antiprotozoal, chemopreventive, anti-inflammatory, antimalarial, anti-flu, antiviral, enzyme inhibitor, anti- hypercholesterolemic |
|
23.31 |
(-)-Nortrachelogenin |
C20H22O7 |
374.1 |
3.55 |
Not known |
|
24.21 |
5,8,11,14-Eicosatetraynoic acid, methyl ester |
C21H26O2 |
310.2 |
8.97 |
Arachidonic acid Inhibitor, Increases Aromatic Amino acid decarboxylase activity, Inhibits production of uric acid, Urine acidifier, Catechol O Methyl transferase inhibitor, Methyl donor, Methyl guanidine inhibitor, Acidifier |
|
26.96 |
Hexadecanoic acid, 1a,2,5,5a,6,9,10,10a-octahydro-5,5a- dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1H-2,8a- methanocyclopenta[a]cyclopropa[e]cyclodecen-6-yl ester, [1aR- (1a.alpha.,2.alpha.,5.beta.,5a.beta.,6.beta.,8a.alpha.,9.alpha.,10 a.alpha.)]- |
C36H58O6 |
586.4 |
2.29 |
Not known |
|
27.25 |
Dodecanoic acid, 1a,2,5,5a,6,9,10,10a-octahydro-5a-hydroxy-4- (hydroxymethyl)-1,1,7,9-tetramethyl-6,11-dioxo-1H-2,8a- methanocyclopenta[a]cyclopropa[e]cyclodecen-5-yl ester, [1aR- (1a.alpha.,2.alpha.,5.beta.,5a.beta.,8a.alpha.,9.alpha.,10a.alpha. )]- |
C32H48O6 |
528.3 |
2.01 |
Not known |
|
28.11 |
Hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester |
C35H68O5 |
568.5 |
3.47 |
Arachidonic acid Inhibitor, Increases Aromatic Amino acid decarboxylase activity, Inhibit production of uric acid, Urine acidifier, |
|
29.51 |
9-Octadecenoic acid, 1,2,3-propanetriyl ester, (E,E,E)- |
C57H104O6 |
884.8 |
19.20 |
Not known |
GC MS Analysis
Figure 2 displays the findings of the GC-MS analysis of polyherbal decoction. It reveals the presence of volatile and semi-volatile compounds in the formulation, which can provide insight into its bioactive properties. The following metabolites have been identified and presented in
The chemicals identified in the GC-MS study include Benzoic acid, 11-Octadecenoic acid (methyl ester), 17-Octadecynoic acid, Gingerol, 1-Heptatriacontanol (corrected), 5,8,11,14-Eicosatetraynoic acid (methyl ester), and Hexadecanoic acid (1-(hydroxymethyl)). Benzoic acid is an antibacterial and preservative essential in pharmaceutical treatments. Methyl 11-Octadecenoate and 17-Octadecynoic acid are fatty acid esters known for their anti-inflammatory, antioxidant, and potentially lipid-lowering characteristics41. These features make them effective in treating inflammatory illnesses.
Gingerol, an active compound found in ginger, is known for its anti-inflammatory, analgesic, and gastroprotective properties42. The presence of this component in polyherbal decoction signifies its efficacy in treating digestive issues and inflammatory diseases. Similarly, 1-Heptatriacontanol is associated with neuroprotection, whereas 5,8,11,14-Eicosatetraynoic acid, methyl ester, has anticancer and cancer-preventive properties43, 44. The chemical molecule, hexadecanoic acid 1-(hydroxymethyl)-1, 2-ethanediyl ester, also known as a palmitic acid derivative, is recognized for its antibacterial, anti-inflammatory, and skin-protective effects.
This demonstrates that polyherbal decoction possesses many medicinal properties, including anti-inflammatory, antibacterial, antioxidant, and digestive system benefits. The plant's traditional usage in Ayurveda and other therapeutic systems is consistent with its efficacy in treating conditions associated with inflammation, infection, and digestive issues. Further research is required to isolate and identify these substances and to conduct a pharmacological assessment of the specific cure within the context of this particular formulation.

Concentration-dependent effects of polyherbal extract compared to ascorbic acid. (A) Comparative graphical representation of ABTS radical scavenging activity of polyherbal decoction and Ascorbic acid. (B) Comparative graphical representation of FRAP assay of polyherbal decoction and Ascorbic acid.

Comparative graphical representation of protein denaturation activity of polyherbal decoction and Diclofenac sodium.
ABTS and FRAP Assay
Ascorbic acid was used as a standard for determining antioxidant activities using the ABTS (Supplementary Table S1) and FRAP assays (Supplementary Table S2). In contrast, Diclofenac sodium was used for the anti-inflammatory assays, including Protein Denaturation Assay and Membrane Stabilization Assay. These assays were performed to facilitate more effective comparisons with the test sample polyherbal decoction. The concentration of the polyherbal decoction and the standard used for the antioxidant assays ranged from 5 to 320 µg/mL. However, for the anti-inflammatory assays, the concentrations were between 50 to 1600 µg/ml. All reactions were performed in Triplicate, and the bar graphs were plotted using the average of different concentrations of polyherbal decoction and its standard deviation. The average IC value, or half-maximal inhibitory concentration, was obtained by converting the concentration against the percentage inhibition or activity values using the graph's R² regression trend line equation.
Figure 3A shows that the IC value for the ABTS radical scavenging activity of polyherbal decoction was 170.93 µg/ml, and that of the control was 17.14 µg/ml for ascorbic acid, it can be concluded that the amount of radical scavenging activity in the polyherbal decoction is more than 10-fold less than the standard. As shown in Figure 3B, the test sample and the standard for the FRAP assay both exhibited increased activity with increasing concentrations. More importantly, polyherbal decoction had higher FRAP activity than ascorbic acid.
Protein Stabilization Study
Figure 4 illustrates the protein denaturation activity of the polyherbal decoction at various concentrations, ranging from 50 µg/ml to 1600 µg/ml, compared to Diclofenac Sodium. The anti-inflammatory action is assessed based on the degree of protein denaturation. The level of inhibition is measured as a percentage of the control, with a higher percentage indicating a more substantial anti-inflammatory impact. At a 50 µg/ml concentration, Diclofenac sodium exhibits more action than the polyherbal decoction in inhibiting denaturation, with a difference of approximately 10%. As the concentration increases, both chemicals exhibit similar heightened activity. However, Diclofenac sodium consistently exhibits higher levels of inhibition at various concentrations.
At a concentration of 200 µg/ml, the activity of the polyherbal decoction is comparable to that of Diclofenac sodium, with only a slight difference. However, the difference in effectiveness is significantly more significant at higher doses, particularly 800 and 1600 µg/ml (Supplementary Table S3). At a 1600 µg/ml dose, both samples exhibit the most significant inhibition of denaturation. Diclofenac sodium exhibits approximately 90% activity, whereas the polyherbal decoction indicates around 80% activity. Based on the data, it can be inferred that polyherbal decoction can block protein denaturation. However, as measured in this experiment, its anti-inflammatory activity is slightly lower than that of Diclofenac sodium, particularly at higher concentrations. Nevertheless, there is a clear correlation between the concentration of the drug and its effectiveness, indicating that it is a dose-dependent substance. While it may have potential as an anti-inflammatory agent, it is not as powerful as Diclofenac sodium.

Comparative graphical representation of membrane stabilisation activity of polyherbal decoction and Diclofenac sodium.

The dose-response curve represents the relationship between compound dilutions and % cell viability. The y-axis indicates the percentage of cell viability, while the x-axis represents the dilutions on a logarithmic scale. Red data points represent experimental measurements. The half-maximal effective concentration is indicated at approximately 60% viability, as marked by the red dashed lines intersecting the curve.
Membrane Stabilization Study
According to Figure 5, the IC value for the membrane-stabilizing activity of the polyherbal decoction is 836. The IC values of NSAIDs were 56 µg/ml for Flurbiprofen and 378.83 µg/ml for Diclofenac sodium (Supplementary Table S4). This suggests that the polyherbal decoction has a lower membrane-stabilizing activity level than the standard. Based on the findings of the in vitro antioxidant and anti-inflammatory assays, polyherbal decoction demonstrated significant activity. It exhibited more potent antioxidant and anti-inflammatory effects compared to the standard. Nevertheless, the molecule exhibited a membrane-stabilizing activity considerably lower than that of Diclofenac sodium. To summarize, polyherbal decoctions have a lower degree of membrane-stabilizing impact. However, it exhibits significant antioxidant and anti-inflammatory properties, suggesting its potential as a valuable therapeutic agent in medicine.
Cell Growth Inhibition Property
The test items were tested against the A549 (corrected) cell line, and the dilutions of the test items ranged from 1:50 to 1:3200 in a 2-fold serial dilution series. The test compound at each concentration was performed in Triplicate, and cumulative variation was maintained at less than 20% between the data points. Figure 6 illustrates the effect of polyherbal decoction on the viability of A549 cells, a line of human lung cancer cells, at various dilutions. The x-axis represents the dilutions of polyherbal decoction on a logarithmic scale, while the y-axis shows the percentage of cell viability. The curve follows a typical sigmoidal dose-response pattern, indicating a concentration-dependent effect of polyherbal decoction on cell viability.
Polyherbal decoction exhibits a minimal effect at lower dilutions, with viability remaining above 80%. As the dilution decreases (indicating higher treatment concentrations), cell viability drops significantly, indicating a cytotoxic effect on the A549 cells. The IC value, which represents the concentration of polyherbal decoction required to reduce cell viability by 50%, is visually identified by the intersection of the dotted red lines. The curve steeply declines around this point, confirming that the treatment becomes increasingly effective at reducing viability as the concentration increases.
At higher concentrations (lower dilutions), the curve plateaus, indicating that the maximum cytotoxic effect of the polyherbal decoction has been reached, with viability dropping to approximately 20-30%. The results suggest that polyherbal decoction exhibits a notable dose-dependent cytotoxic effect on A549 cells, indicating potential anticancer properties. Further experiments would be necessary to determine the mechanism of action and explore its effectiveness in other cancer cell lines.

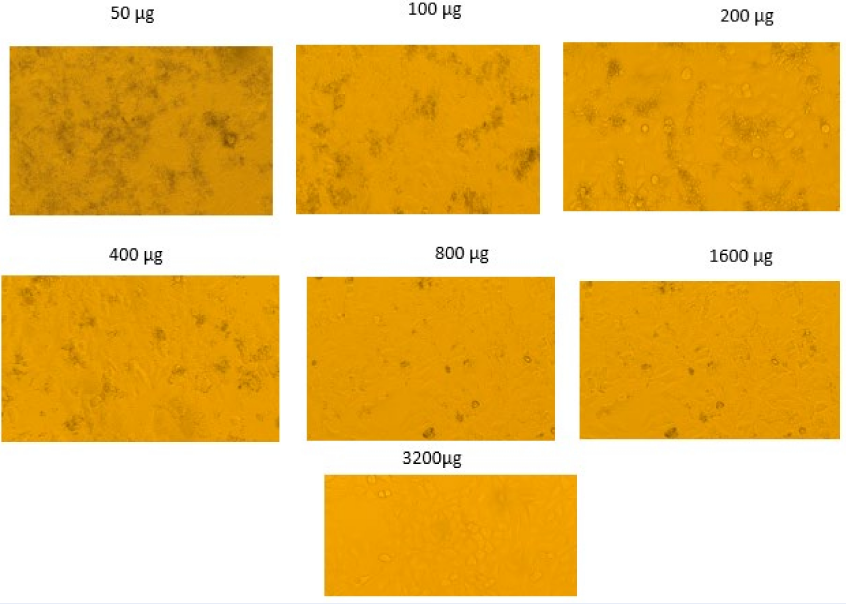
Microscopic images showing the effect of varying concentrations of a compound (ranging from 50 µg to 3200 µg) on cell morphology. As the concentration increases, changes in cell density and morphology are observed. The images reveal a dose-dependent response, with higher concentrations showing more pronounced cellular changes.
Cytotoxicity/Anticancer Assay
Figure 7 displays a series of images illustrating the outcomes of the MTT experiment conducted on cells treated with varying drug concentrations, ranging from 50 to 3200 µg. The MTT assay is used to assess cell viability by measuring mitochondrial activity. In this assay, living cells convert MTT into a formazan product, which appears as black granules. The extent of formazan production measures the number of viable cells resulting from the reduction of tetrazolium salt. Each panel in Figure 7 indicates a distinct concentration of the drug applied to the cells, which can be used to evaluate cytotoxicity. At concentrations of 50 µg, 100 µg, and 200 µg, the cells exhibit black patches, indicating their metabolic activity and viability. Nevertheless, as the concentration was augmented from 400 µg to 3200 µg, there appears to be a decrease in staining intensity, indicating a reduction in viable cells.
At a concentration of 3200 µg, the cells appear to have the least staining, indicating that the drug has likely produced cytotoxicity at this dosage, resulting in a low level of cell viability. The observed amount of inhibition is characteristic of cytotoxicity studies. As the concentration of the chemical increases, the level of cell survival falls in a dose-dependent manner. Based on the acquired data, it may be inferred that there is a correlation between higher concentrations of the chemical and decreased cell viability. The MTT assay, as depicted in the picture, is valuable for quantifying cytotoxicity across various concentration levels. However, to determine the precise level of cytotoxicity and other variables, it is essential to conduct further quantitative assessments and determine the IC50, which refers to the concentration of the poisonous substance that enables fifty percent of the cells to survive.
Molecular Docking
The molecular docking studies highlight phytochemicals that target the protein structure with PDB ID 3IL8, related to irritable bowel syndrome (IBS). The three compounds, Hexadecanoic acid, Gingerol, and Benzoic acid, along with their corresponding binding energies, demonstrate their potential as therapeutic agents against this IBS-related protein target. Hexadecanoic acid exhibits the most favorable interaction with the 3IL8 protein, indicating the lowest binding energy of −9.865 kcal/mol. The negative result indicates a substantial binding affinity, suggesting that Hexadecanoic acid may effectively regulate the activity of this protein. This discovery identifies hexadecanoic acid as a promising subject for additional research on IBS treatment. Gingerol, recognized for its anti-inflammatory and gastrointestinal advantages, demonstrates a notable binding affinity with a binding energy of −9.114 kcal/mol. Although marginally less potent than Hexadecanoic acid, the binding energy suggests a robust interaction, indicating that Gingerol may be a promising candidate for investigation of its therapeutic potential in IBS. The advantageous binding affinity of Gingerol corresponds with its established therapeutic effects, especially in mitigating gastrointestinal distress, a primary symptom of IBS. Benzoic acid exhibits a binding energy of −8.666 kcal/mol, which, although marginally weaker than that of the other two chemicals, nonetheless signifies a significant interaction with the protein target. The binding potential of benzoic acid indicates its possible role in treatment techniques, although it may be less successful than hexadecanoic acid or Gingerol when considering binding energies alone.
Binding energy values (in kcal/mol) of selected phytochemicals from polyherbal decoction
|
S. No |
Phytochemicals |
Binding Energy (Kcal/mol) |
|---|---|---|
|
1 |
Hexadecanoic acid |
-9.86 |
|
2 |
Gingerol |
-9.11 |
|
3 |
Benzoic acid |
-8.66 |

The three-dimension complex of Interleukin8-phytocompounds modeled by Autodock Vina is presented: IL8- Hexadecanoic acid (A), IL8-Benzoic acid (B), and IL8-Gingerol (C), respectively. Chemical interaction diagrams of Hexadecanoic acid (D), Benzoic acid (E), and Gingerol (F) with the amino acids of the IL8 were modeled.
The illustration depicts the molecular docking interactions of Interleukin-8 (IL-8) with three phytocompounds: hexadecanoic acid, benzoic acid, and Gingerol, utilizing the AutoDock Vina program. Figure 8 (A, B, and C) depicts the space-filling models of IL-8 complexed with hexadecanoic acid, benzoic acid, and Gingerol, respectively. Schemes (D), (E), and (F) illustrate the chemical interaction mechanisms that delineate the specific amino acid residues responsible for the binding of each specified phytocompound to the IL-8 protein. Panel A demonstrates that the Hexadecanoic acid molecule aligns with the IL-8 protein, effectively occupying the active site and exhibiting substantial van der Waals contacts. The chemical interaction diagram (Panel D) indicates that Hexadecanoic acid establishes hydrogen bonds with residues THR (Thr) A:12 and GLU (Glu) A:38. It also participates in hydrophobic interactions with CYS (Cys) A:9 and ILE (Ile) A:10, the mutation of which enhances the binding of the area to the protein. The interactions suggest that Hexadecanoic acid maintains a persistent binding conformation within the active site of IL-8, correlating with elevated binding energy, as demonstrated in this study and other research.
Figure 8B illustrates the docking orientation of Benzoic acid engaging with the IL-8 protein target, indicating that it binds to a distinct location compared to Hexadecanoic acid. The chemical interaction diagram in Panel E demonstrates that Benzoic acid interacts with amino acids, including THR A:12, GLU A:38, and CYS A:7. Significantly, it also engages in π-π stacking interactions with aromatic residues, which can augment its moderate binding affinity. Both hydrophilic and hydrophobic components indicate that Benzoic acid's capacity to engage with IL-8 activity is mediated through multiple mechanisms. The final set of interactions is illustrated in Panel C, where Gingerol demonstrates a degree of contact with the IL-8 protein. Panel F illustrates that Gingerol establishes multiple hydrogen bonds with critical residues, specifically ASP (Asp) A:52 and LYS (Lys) A:54, while also engaging in hydrophobic interactions with PRO (Pro) A:53 and CYS (Cys) A:9. The interactions validate Gingerol's demonstrated high binding energy, as indicated by the competitive binding energy value. Hydrogen bonding and hydrophobic interactions suggest that Gingerol may significantly influence IL-8 activity, perhaps due to its anti-inflammatory properties.

Root Mean Square Deviation of Interleukin8-phytocompounds is presented (A): Root Mean Square Deviation of a complex of Interleukin8-phytocompounds (B).
Molecular Dynamics Simulations
Figure 9 displays molecular dynamics (MD) simulation findings, examining the stability and flexibility of the Interleukin-8 (IL-8) protein complexed with four distinct compounds: Entospletinib, Hexadecanoic acid, Benzoic acid, and Gingerol. The initial plot illustrates the temporal variation of the Root Mean Square Deviation (RMSD) of the protein-ligand complexes during the simulation duration of 50,000 picoseconds. The second plot depicts the Root Mean Square Fluctuation (RMSF) of the protein residues for each combination.
The RMSD plot (upper panel) illustrates the stability of the protein-ligand complexes during the simulation investigation. The calculation of RMSD at lower and less variable values indicates a more robust and stable binding of the ligand to the protein. Entospletinib exhibits the lowest RMSD among the four complexes, remaining below 0.3 Å, signifying a strong binding affinity with IL-8. Hexadecanoic acid (orange line) exhibits moderate stability, with little alterations, indicating that the structural conformation of the binding site remains relatively stable during the simulation. Concurrently, Benzoic acid (gray line) and Gingerol (yellow line) exhibit marginally elevated and more oscillatory RMSD profiles, with Gingerol demonstrating significant variations, indicating an unstable binding relationship with the IL-8 protein. These data suggest that Gingerol exhibits greater structural flexibility, as evidenced by elevated RMSD values of the protein density map, which may influence binding affinity.
The RMSF plot (bottom panel) indicated the mobility of amino acid residues within the protein, which is more significant in the flexible regions. Entospletinib yields reduced RMSF values for nearly all residues, indicating a stiff conformation in the protein-ligand interaction. This aligns with the previously observed constant RMSD values. This confirms that Entospletinib facilitates a robust and persistent binding affinity with IL-8. The current RMSF values are equivalent to those of hexadecanoic acid, exhibiting a moderate value along the protein's length, with slightly increased flexibility in specific areas, including the C-terminal residues. The two ligands exhibit elevated RMSF peaks at positions 55-75 of the protein. The increased diversity of these areas suggests that the IL-8 protein undergoes structural alterations upon interacting with its partner protein, potentially leading to a more dynamic binding process.
The integrated RMSD and RMSF analyses provide insight into the interaction of the four ligands with IL-8. Entospletinib exhibits the highest rigidity and stability among the complexes' structures, indicating a significant potential to control IL-8 activity. Hexadecanoic acid exhibits commendable stability, whereas Benzoic acid and Gingerol demonstrate greater flexibility, potentially elucidating their therapeutic efficacy. The results indicate that all four molecules interact with IL-8; however, Entospletinib and Hexadecanoic acid may exhibit stable binding, presenting potential as therapeutic options for future IL-8-associated inflammatory illnesses, including IBS.
Discussion
The results of this study support the medicinal application of polyherbal decoctions and their bioactive constituents, as determined through qualitative and quantitative analyses using phytochemical, chromatographic, antioxidant, anti-inflammatory, and cytotoxicity investigations. The initial phytochemical screening revealed the existence of various categories of biologically active chemicals, including alkaloids, carbohydrates, glycosides, flavonoids, phytosterols, amino acids, and tannins. These chemicals are utilized for their pharmacological properties, specifically their anti-inflammatory, antioxidant, antibacterial, and tissue healing activities45. It is essential to note the influence of alkaloids and flavonoids on altering the anti-inflammatory and antioxidant effects of polyherbal decoctions. This aligns with its historic use in treating inflammatory conditions and promoting overall health46. The absence of quinones, terpenoids, and other chemicals suggests that the therapeutic properties of this formulation can be attributed to the discovered bioactive molecules.
The HPTLC examination provided further information about the chemical composition of polyherbal decoction. It revealed the presence of twelve distinct peaks, with two main ingredients identified as Compound A and Compound B. The value of 68 suggests that this chemical may be the primary contributor to the biological activity observed in this study. However, additional minor components, such as Compound A, indicate that the formulation may contain many active ingredients that interact to provide therapeutic effects. It is essential to conduct additional investigations involving mass spectrometry and nuclear magnetic resonance to gain a more comprehensive understanding of these compounds, including their identity and therapeutic use47.
The GC-MS findings in this study further supported the HPTLC results by confirming the existence of many volatile and semi-volatile substances with pharmacological properties. Several chemicals found include benzoic acid, Gingerol, and fatty acid esters. These compounds are known to possess anti-inflammatory, antioxidant, and antibacterial activities. Combining these compounds and other compounds identified in the investigation provides a solid foundation for using polyherbal decoction in treating inflammation, infection, and related ailments. The presence of gingerol specifically enhances the gastroprotective and anti-inflammatory effects of the formulation, which is traditionally used to promote digestive health48.
The subsequent study conducted in a laboratory setting confirmed the efficacy of polyherbal decoction in terms of its antioxidant and anti-inflammatory properties. The ABTS and FRAP assays revealed antioxidant activity that was significantly lower than that of ascorbic acid. Nevertheless, the formulation exhibited an activity that increased proportionately to the concentration, suggesting a notable antioxidant activity in higher-concentration solutions. The protein stabilization and membrane stabilization assays demonstrated the anti-inflammatory effect of the polyherbal decoction, albeit with slightly lesser efficacy than typical NSAIDs, such as Diclofenac sodium. Considering the dose-dependent nature of these effects, higher formulation concentrations may offer significant therapeutic advantages in treating inflammatory disorders.
The cytotoxicity data collected from the A549 cell line, which consists of human lung cancer cells, provided valuable information regarding the potential use of polyherbal decoction as an anticancer treatment. The MTT experiment findings indicated a drop in cell viability as the concentration increased, and higher concentrations led to a notable decrease in metabolic activity. The decline in cell viability as the concentration of the formulation increases indicates that the formulation possesses cytotoxic qualities that can be harnessed for cancer treatment. However, further empirical investigation is necessary to elucidate the mechanism of action, identify the specific constituents of the plant responsible for this effect, and assess the impact of this therapy on various types of cancer cells.
Molecular docking and molecular dynamics (MD) simulation analyses have been employed for the IL-8 protein in conjunction with four ligands, Hexadecanoic acid, Gingerol, Benzoic acid, and Entospletinib, associated with IBS. The molecular docking tests indicated that hexadecanoic acid exhibited the highest binding affinity with IL-8, evidenced by the lowest binding energy of -9.865 kcal/mol, suggesting its potential as a treatment agent in IBS. The binding energy of gingerol was determined to be -9.114 kcal/mol, which aligns with its role as an anti-inflammatory agent and in treating gastrointestinal disorders. Benzoic acid exhibited a somewhat reduced binding energy of -8.666 kcal/mol, indicating a weaker binding, while it remained significant. The MD simulations revealed that Entospletinib exhibited superior binding stability, characterized by low RMSD and RMSF values, indicating an optimal interaction with IL-8. Similarly, hexadecanoic acid exhibited stability during simulations, whereas benzoic acid and Gingerol showed comparatively greater flexibility, indicative of a dynamic interaction. In conclusion, our investigation supports the use of Entospletinib and Hexadecanoic acid as therapeutic agents for modifying IL-8 in IBS and other inflammatory diseases.
Hence, the results of this study unambiguously support the therapeutic importance of polyherbal decoctions, considering their traditional applications in treating inflammatory diseases, oxidative stress, and gastrointestinal problems49. While the formulation may not be as potent as synthetic medications like Diclofenac sodium, its biological actions suggest that it could be used alongside or as an alternative to synthetic drugs, particularly in cases where natural treatments are preferred. Isolating the primary bioactive chemicals, particularly those with anti-inflammatory, antioxidant, and cytotoxic properties, enables a deeper understanding of the pharmacological capabilities of polyherbal decoctions. Additional research is needed to identify these compounds, conduct thorough examinations based on their mechanisms, and assess the practical value of these molecules in clinical settings.
Conclusions
This research highlights the several functions of polyherbal decoction, an Ayurvedic medication, which include anti-inflammatory, antioxidant, and cytotoxic properties. The preliminary qualitative study also revealed the existence of crucial secondary metabolites, including alkaloids, flavonoids, and phytosterols, which possess medicinal properties. The HPTLC and GC-MS analyses provided additional insight into identifying many volatile and non-volatile chemicals with well-established pharmacological characteristics, such as benzoic acid and gingerol. The studies provided further evidence of the formulation’s antioxidant and anti-inflammatory properties; however, its activity was often lower than that of commercially manufactured medicines, such as Diclofenac sodium. In addition, the MTT assay demonstrated that the chemical had a cytotoxic effect on A549 lung cancer cells, with the extent of toxicity increasing with concentration. This suggests that the compound has exhibited preliminary cytotoxic activity in A549 cells, indicating a need for further investigation to evaluate its potential anticancer effects across additional models. While the formulation may not be as potent as synthetic medications, its natural origins and various biological actions make it a viable option for complementary therapy. Further research is necessary to identify active chemicals and assess their efficacy in medicine.
Abbreviations
ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)), ANOVA (Analysis of Variance), ATS 4 (Automatic TLC Sampler 4), CO(Carbon Dioxide), Da (Dalton), DMEM (Dulbecco's Modified Eagle Medium), DMSO (Dimethyl Sulfoxide), DNA (Deoxyribonucleic Acid), DPPH (2,2-Diphenyl-1-picrylhydrazyl - implied), eV (Electron Volt), FBS (Fetal Bovine Serum), F254 (Fluorescence indicator at 254 nm), FRAP (Ferric Reducing Antioxidant Power), GC-MS (Gas Chromatography-Mass Spectrometry), GI₅₀ (50% Growth Inhibition), GROMACS (GROningen MAchine for Chemical Simulations), HPTLC (High-Performance Thin Layer Chromatography), HCl (Hydrochloric Acid), IBS (Irritable Bowel Syndrome), IC₅₀ (Half-Maximal Inhibitory Concentration), ID (Internal Diameter), IL-8 (Interleukin-8), MD (Molecular Dynamics), MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), Na (Sodium), Na₂S₂O (Sodium Metabisulfite), NCCS (National Centre for Cell Science), NIST (National Institute of Standards and Technology), NPT (Constant Number of particles, Pressure, Temperature), NVT (Constant Number of particles, Volume, Temperature), OD (Optical Density), PBS (Phosphate-Buffered Saline), PDB (Protein Data Bank), RBC (Red Blood Cell), Rf (Retention Factor), RPM (Revolutions Per Minute), RMSD (Root Mean Square Deviation), RMSF (Root Mean Square Fluctuation), SEM (Standard Error of the Mean), SPC (Simple Point Charge water model), TIC (Total Ion Current), TLC (Thin Layer Chromatography), TPTZ (2,4,6-Tripyridyl-s-triazine), UV (Ultraviolet), v/v (Volume per Volume), µg/mL (Micrograms per Milliliter), µL (Microliter), and °C (Degrees Celsius)
Acknowledgments
None.
Author’s contributions
Conceptualization and methodology: LR, KS, KMS; Investigation: LR, KP, AP, KMS; Analysis, LR, KS, RS, TA; Writing: LR, TA, KMS; Revise: TA, KMS; All authors have read and agreed to the final version of the manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
The study protocol was approved by the Institutional Review Board (or Ethics Committee) of Bharath Institute of Higher Education and Research, Chennai (Protocol code BIHER/BSCI/IHEC/2021/15 dated 27.11.2021).
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

