Recent Advancement, Mechanisms of Action and Applications of Tumor-Targeting Peptides
- Department of Precision Medicine, University of Campania, Luigi Vanvitelli, Naples, Italy
- Centre for Applied Molecular Biology, 87-West canal, Bank Road, University of the Punjab, Lahore-53700, Pakistan
Abstract
Tumor targeting peptides (TTPs) have emerged as new therapeutic and diagnostic tools in oncology, due to their low immunogenicity, high specificity, and ability to efficiently penetrate tumor cells and tissues. They exert their effects using various mechanisms such as receptor-mediated targeting, cell-penetrating properties, and enzyme-responsive activation, allowing selective delivery of drugs, nanoparticles, and imaging agents to cancer cells. Advances in peptide engineering, such as D-amino acid incorporation, cyclization, and multivalent designs, have substantially enhanced their stability, affinity, and bioavailability. They are widely utilized in immunotherapy, precision imaging, and targeted drug delivery, thus improving cancer detection and outcomes. Recent developments, including peptide–drug conjugates, hybrid peptide–nanoparticle systems, and peptide-based immune modulators, have significantly broadened the clinical potential of TTPs. This review highlights the fundamental mechanisms, therapeutic applications, and cutting-edge advancements in TTPs, underscoring their role in personalized cancer therapy.
Introduction
Peptides are short chains of amino acids, consisting of 2-50 amino acids, linked by peptide bonds. They play a crucial role in biological processes and have a wide range of applications in medicine, biotechnology, and research1. Many peptides function as hormones, regulating various physiological processes (, insulin, glucagon). Some act as neurotransmitters, transmitting signals in the nervous system (., endorphins)2. Some peptides have antimicrobial activity, serving as natural antibiotics (., defensins). The therapeutic potential of peptides is vast, ranging from cancer treatment and management of metabolic disorders to antiviral therapies and vaccine development3. Advances in peptide synthesis, such as solid-phase peptide synthesis and automated synthesis, have significantly enhanced their production efficiency4, 5. Furthermore, peptide modifications and delivery systems have improved their stability and bioavailability. They also serve as valuable diagnostic tools, contributing to fields such as protein–protein interactions and biomarker identification6. Their applications extend to cosmetics, where they promote collagen production and wound healing7. Despite challenges such as cost-effective production, ongoing innovation in peptide technology continues to expand their utility in medicine, biotechnology, and beyond8.
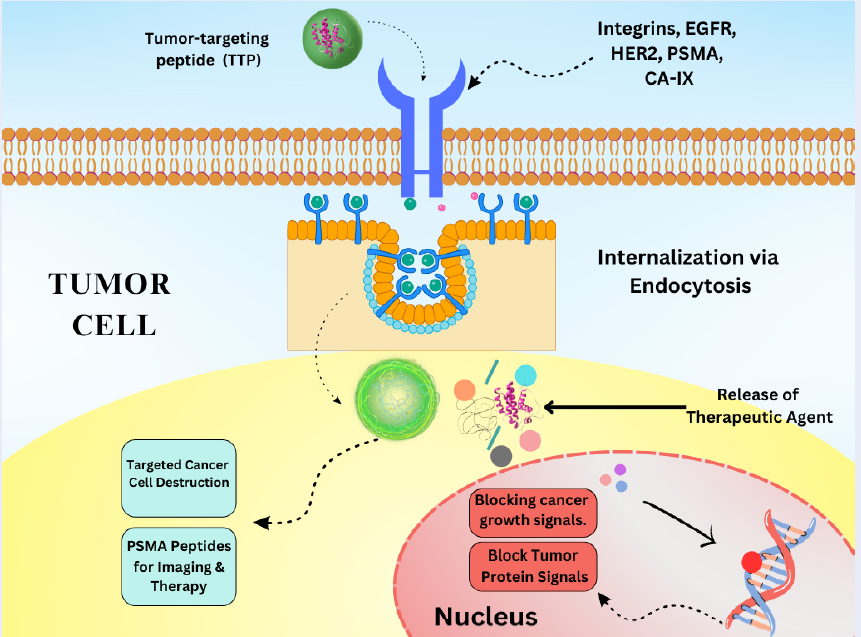
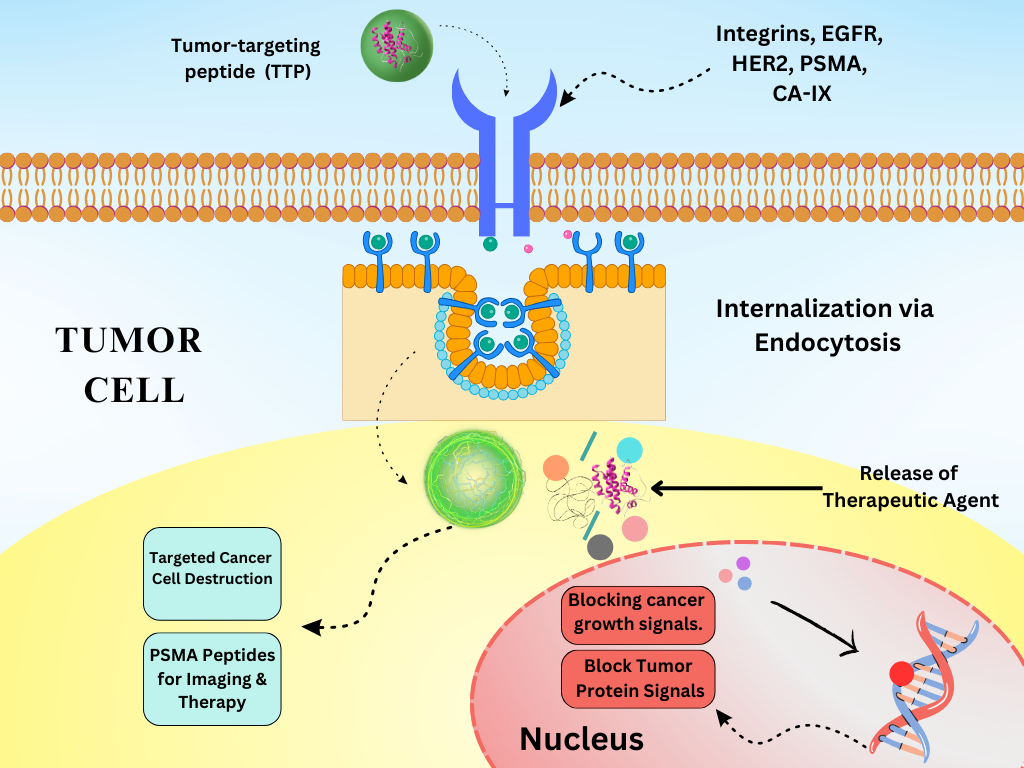
Peptides can be designed to bind specifically to target molecules, making them highly specific in their action. Generally, peptides have lower toxicity than small-molecule drugs9. Peptides can be easily modified to enhance their stability and activity. They have emerged as promising agents in cancer treatment due to their ability to specifically target cancer cells, modulate the immune response, and deliver therapeutic payloads10. Their versatility and precision make them valuable for developing targeted therapies compared to traditional chemotherapies. Peptides can target specific receptors on cancer cells, delivering cytotoxic agents or inhibiting tumor growth11. Peptides can be conjugated to cytotoxic drugs, directing these drugs specifically to cancer cells, thereby minimizing the damage to healthy cells12. The peptide sequence binds to receptors overexpressed on cancer cells, allowing the drug to be directly released at the cancer site. Peptides designed to bind to tumor-specific antigens or receptors (, EGFR, HER2) enhance the delivery of therapeutic agents (Figure 1 )13. Peptides targeting integrin receptors overexpressed in tumors can deliver imaging or therapeutic agents14. Peptides derived from tumor antigens can be used to stimulate the immune system to recognize and attack cancer cells15. Some peptides have inherent cytotoxic properties, inducing apoptosis and disrupting cancer cell membranes (Figure 1 )16.
Schematic representation of TTP mechanisms: Receptor-mediated targeting, Cell-penetrating peptide internalization, Enzyme-responsive activation.
Mechanism of Tumor Targeting Peptides
Receptor-Mediated Targeting by TTPs for Facilitating Delivery
Receptor-mediated targeting by TTPs leverages the overexpression of specific receptors on the tumor surface. These peptides are specifically designed to bind these receptors, facilitating the targeted delivery of therapeutic agents, imaging compounds, and diagnostic markers directly to the tumor site17. On binding to their target receptor, these peptides can facilitate the internalization of the peptide–receptor complex, allowing for intracellular delivery of therapeutic agents (Figure 2 )18. TTPs are engineered to bind with high affinity and specificity to the receptors that are overexpressed on tumor cells19. This selective binding ensures that the peptide is delivered to tumor cells, sparing healthy tissues. Upon receptor binding, the peptide–receptor complex is internalized by the cancer cell through endocytosis. This internalization allows the payload to be delivered directly into cancer cells, thereby enhancing therapeutic efficiency (Figure 2 )20. Once inside the cell, the therapeutic agent (, drug, toxin, or gene therapy vector) is released, where it can exert its intended effect21.
Mechanism of receptor-mediated endocytosis: TTP binds to overexpressed receptors, Internalization via clathrin-coated vesicles, Endosomal escape, Intracellular release of therapeutic payload.
Most notably, clathrin-mediated endocytosis is the predominant route for internalization of receptor–ligand complexes in most mammalian cells. In CME, receptor complexes accumulate in clathrin-coated pits (~100–150 nm in diameter), where adaptor proteins (., AP-2) recruit clathrin triskelia to form a coated vesicle. Dynamin then pinches off the vesicle, which uncoats and fuses with early endosomes22. Vesicles of this size (~100 nm) are well-suited for the bulk uptake of peptide–drug conjugates. Typically, acidification within late endosomes and lysosomes promotes cargo release, but also risks enzymatic degradation; thus, TTP designs often incorporate endosomal escape motifs to ensure payload release into the cytosol before lysosomal degradation23. Moreover, caveolin-mediated endocytosis occurs via flask-shaped caveolae (~60–80 nm in diameter) enriched in caveolin-1 and Cavin proteins. Ligand–receptor binding induces caveolar budding in a dynamin-dependent manner, forming caveolar carriers that bypass early endosomes and lysosomes, often trafficking to caveosomes or the Golgi and endoplasmic reticulum24. The smaller vesicle size and nonacidic routing protect sensitive cargo (., peptides, proteins, nucleic acids) from degradation, but may slow release kinetics, necessitating specialized release triggers in TTP designs25.
Implications for TTP Design and Drug Release
For CME-internalized cargos, engineering pH-sensitive or membrane-disruptive elements (., histidine-rich sequences) can accelerate endosomal escape, thus maximizing cytosolic delivery before lysosomal degradation26. CvME avoids lysosomes, thereby protecting delicate agents like siRNA and proteins from degradation. However, because it operates more slowly, special linkers responsive to specific signals (., redox-sensitive disulfides) may be required to release cargos at the optimal time27. Targeted trafficking and differential routing can be leveraged to direct payloads to specific intracellular organelles; for example, CvME-mediated trafficking to the ER favors the delivery of unfolded protein therapeutics28.
This process ensures that the cytotoxic effect remains confined to cancer cells, thereby reducing systemic side effects29. Some common receptors targeted by tumor-targeting peptides are integrins, which are involved in tumor angiogenesis and metastasis, thus making them highly effective targets for TTPs. For example, RGD peptides (arginine-glycine-aspartic acid) specifically target these integrins to deliver therapeutic agents and imaging compounds30. EGFR, which is overexpressed in various cancer types, is targeted by peptides to inhibit growth signals and deliver cytotoxic agents. Peptides that bind EGFR can deliver chemotherapeutic drugs specifically to EGFR-expressing tumor cells31. Similarly, HER2 is commonly overexpressed in breast cancer and other tumor types, facilitating the effective delivery of therapeutic agents by HER2-targeting peptides32. Folate receptors are overexpressed in certain cancers, making folate-conjugated peptides useful for targeted drug delivery. Moreover, folate-linked peptides facilitate the delivery of chemotherapy drugs to folate receptor-positive tumors33. Prostate-specific membrane antigen (PSMA) is highly expressed in prostate cancer cells, making it an ideal target for peptide-based delivery systems34. Peptides targeting PSMA can deliver radiolabeled compounds for imaging or therapeutic agents, enabling targeted treatment35. Carbonic anhydrase IX (CA-IX) is overexpressed in hypoxic tumors and can be targeted by peptides to deliver therapeutic agents or imaging probes36.
Targeting the Tumor Microenvironment
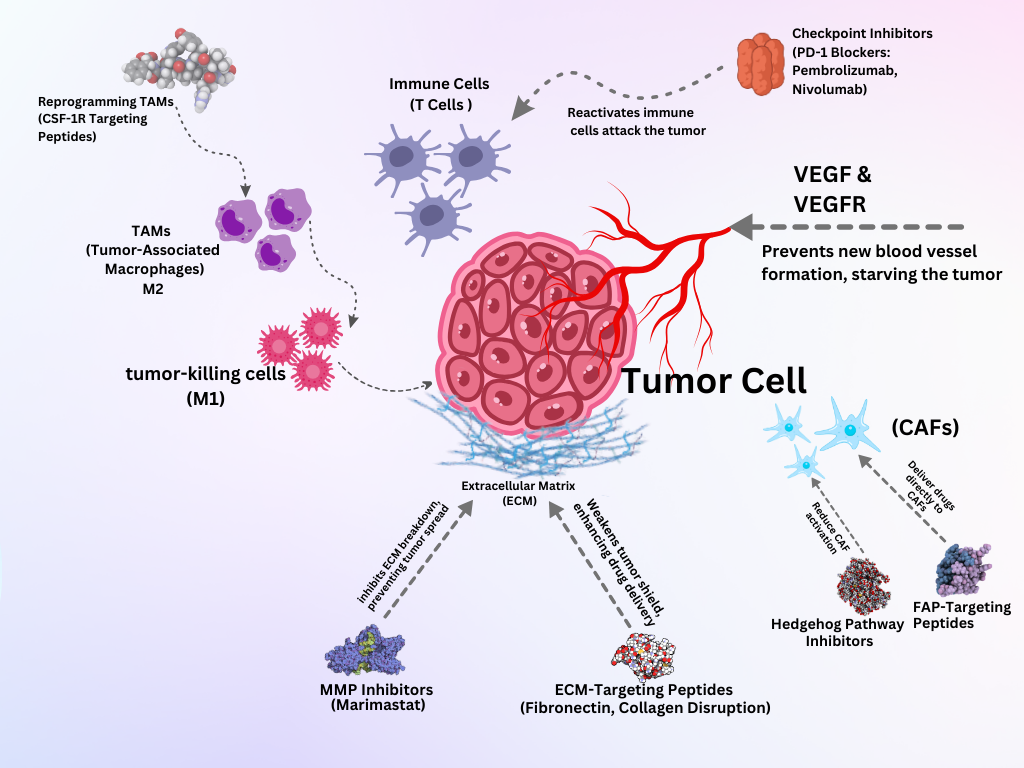
The tumor microenvironment (TME) is a highly complex, adaptive system comprising malignant cells, immune cells, stromal elements, blood vessels, and extracellular matrix (ECM) components. It not only drives tumor growth but also significantly contributes to therapeutic resistance, immune evasion, and metastasis37. Acknowledging the TME's active role in tumor biology has led to the development of therapeutic strategies aiming to disrupt its supportive functions, including vascular normalization, immune response reprogramming, and ECM remodeling, ultimately enhancing the efficacy of conventional therapies38. A key factor underlying the complexity of the TME is the genetic and phenotypic heterogeneity within tumor cell populations. This diversity enables cancer cells to interact with surrounding stromal components via distinct paracrine signaling pathways, which shape their behavior and further promote treatment resistance. The influence of this heterogeneity extends to various stromal cells, including cancer-associated fibroblasts (CAFs), which respond to tumor-derived signals and contribute to ECM remodeling, immune modulation, and therapy resistance39. Although CAFs represent a substantial stromal population, they are part of a broader cellular network that includes endothelial cells, pericytes, and immune infiltrates. Endothelial cells form blood vessels that sustain tumor growth and enable metastatic dissemination. Working in concert, TME components ensure that the tumor remains protected and fully functional40. A more detailed discussion of CAF biology and its therapeutic implications appears in Section 3.3. Here, the focus remains on emphasizing the TME as a whole, underscoring the need for integrated therapeutic approaches that target both tumor cells and their supportive ecosystem to overcome resistance and improve clinical outcomes41.
Strategies for Targeting the Tumor Microenvironment
Inhibiting Angiogenesis
Inhibiting angiogenesis is a crucial strategy in cancer therapy that aims to starve the tumor of the blood supply essential for its growth and metastasis42. Tumor-targeting peptides can be designed to specifically bind to angiogenic markers on endothelial cells, thereby delivering therapeutic agents that inhibit the formation of new blood vessels43. This targeted approach ensures that anti-angiogenic treatments are delivered precisely where they are needed, helping reduce systemic toxicity and optimizing therapeutic efficiency44. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) are key regulators of angiogenesis45. TTPs can be designed to bind to VEGF or VEGFR, blocking their interaction and inhibiting the angiogenic signaling pathway. For example, Bevacizumab (Avastin) is a monoclonal antibody against VEGF46.
Modulating Immune Response
An immunosuppressive environment persists in most tumors, leading to a diminished immune response and allowing cancer cells to remain undetected due to myeloid-derived suppressor cells, regulatory T cells, tumor-associated macrophages, and inhibitory cytokines47. Patients experiencing these immunosuppressive effects can be treated with immune checkpoint inhibitors, which remove inhibitory signals on T cells and allow them to fight the tumor again. When PD-L1 or PD-L2 bind to the PD-1 receptor on activated T cells, the cells become exhausted, and robust immune responses are halted. Pembrolizumab (Keytruda) is a humanized IgG4 monoclonal antibody that binds the programmed cell death-1 (PD-1) receptor on activated T cells and prevents its interaction with PD-L1 and PD-L2, restoring T-cell proliferation and cytotoxicity against tumor cells48. Nivolumab likewise targets PD-1 to release the PD-1–mediated brake on T cells, and has demonstrated clinical efficacy across multiple advanced malignancies by enhancing T-cell–mediated tumor cell killing49.
Therapeutic cancer vaccines represent another modality to stimulate antitumor immunity by presenting tumor antigens to a patient’s antigen-presenting cells. Sipuleucel-T (Provenge) is an FDA-approved autologous cellular vaccine for metastatic prostate cancer in which a patient’s dendritic cells are harvested, incubated with a fusion protein of prostatic acid phosphatase and granulocyte–macrophage colony-stimulating factor, and then reinfused to elicit a sustained, antigen-specific T-cell response and prolong overall survival50. Finally, reprogramming of tumor-associated macrophages from a pro-tumorigenic M2-like state to an antitumorigenic M1-like phenotype can be achieved by targeting the colony-stimulating factor-1 receptor (CSF-1R). Small-molecule inhibitors or peptides against CSF-1R deplete or re-educate M2 macrophages, enhancing antigen presentation and fostering a pro-inflammatory microenvironment conducive to tumor rejection51.
Targeting Cancer-Associated Fibroblasts (CAFs)
Beyond directly targeting cancer cells, tumor-targeting peptides are designed to disrupt the tumor-supportive functions of cancer-associated fibroblasts (CAFs), which are essential to tumor progression. Cancer-associated fibroblasts (CAFs) are a major stromal component of the tumor microenvironment (TME) and play a critical role in supporting tumor progression, invasion, angiogenesis, and immune evasion52. With growth factors, cytokines, chemokines, and enzymes, CAFs modify cancer cells’ responses by remodeling components of the ECM. This remodeling helps tumor cells migrate more easily to other parts of the body and can also force some of the drug’s dose to remain in the intestines, thus reducing its effectiveness. In addition to shaping the stroma, CAFs secrete TGF-β, VEGF, and FGFs, thereby promoting faster growth of cancer cells and fostering new blood vessel development. Moreover, cells in the CAF system release cytokines and chemokines that inhibit the immune response against cancer within the body53.
Recent research finds that CAF populations have many different functions. Distinct subtypes such as myofibroblastic CAFs (myCAFs) and inflammatory CAFs (iCAFs) differ in their phenotypic markers and roles. While myCAFs contribute to ECM stiffening through expression of α-smooth muscle actin (α-SMA) and collagen crosslinking enzymes, iCAFs are characterized by the secretion of pro-inflammatory cytokines like IL-6 and CXCL12, which enhance immune evasion and drive tumor growth54. Targeting CAFs therapeutically has become an area of intense investigation. One promising approach involves the use of tumor-targeting peptides (TTPs) that bind selectively to fibroblast activation protein (FAP), a surface protein highly expressed on CAFs. These peptides can serve as carriers for cytotoxic agents or imaging probes, enabling precise delivery to the CAF-rich regions of tumors55. Peptides designed to inhibit key signaling pathways, such as Hedgehog signaling, have also shown potential in reducing CAF activation and tumor-supportive functions. Additionally, efforts are underway to develop peptide-based inhibitors against matrix metalloproteinases (MMPs) and other ECM-modifying enzymes secreted by CAFs, aiming to limit their remodeling activity and improve drug penetration56.
By specifically disrupting CAF functions, these strategies aim to break down the protective stromal barrier that surrounds tumors, reduce resistance to chemotherapy, and enhance overall treatment efficacy. As understanding of CAF heterogeneity continues to evolve, tailored interventions may offer more precise and effective ways to neutralize their tumor-promoting roles57.
Disrupting Extracellular Matrix
Disrupting the extracellular matrix (ECM) within the tumor microenvironment (TME) is a critical strategy in cancer therapy58. Using tumor-targeting peptides (TTPs) against the extracellular matrix can reduce tumor growth and make other treatment methods more effective59. Targeting the ECM with TTPs can inhibit these processes and enhance the effectiveness of other therapies60. Attaching peptides to specific ECM components, such as fibronectin or collagen, can modify tumor development61. Peptides can inhibit matrix metalloproteinases (MMPs), which degrade ECM components and facilitate tumor invasion. For example, peptides mimicking MMP inhibitors, such as marimastat, can help prevent ECM degradation62. Peptides designed to bind specific ECM components can alter a tumor’s structural integrity. For example, peptides targeting fibronectin or collagen in the ECM are notable examples63. Disruption of the extracellular matrix with tumor-targeting peptides offers a multifaceted approach to cancer therapy by interfering with the structural and signaling functions of the ECM that support tumor growth and invasion64.
Exploiting Hypoxia and Acidity
Tumors often develop regions with low oxygen levels due to abnormal blood vessel formation and rapid tumor growth that consumes oxygen more quickly than it can be adequately supplied. Hypoxia triggers the expression of hypoxia-inducible factors (HIFs), which help tumors survive, promote new blood vessel formation, and spread. To capitalize on this feature, TTPs and prodrugs can be tailored to activate specifically in hypoxic areas while remaining inactive under normal oxygen levels, thus reducing damage to healthy tissues. For example, hypoxia-responsive linkers such as 2-nitroimidazole are commonly used. Under low oxygen, nitro-reductase enzymes convert the nitro group into an amino group, triggering drug release65. Azobenzene-based linkers have also been incorporated into antibody drug conjugates (ADCs) for selective drug delivery in hypoxic tumor tissue66.
Prodrugs are inactive compounds that only become active in hypoxic conditions, targeting low-oxygen tumor cells specifically. Hypoxia-responsive peptides are engineered to release their drugs when exposed to low oxygen levels. These peptides are often combined with drugs that are triggered by HIFs or enzymes overexpressed in hypoxia, like nitroimidazole derivatives67. To further extend TTP applications beyond simple ligand–receptor binding, recent designs incorporate stimuli‑responsive linkers and motifs that react specifically to TME cues, most prominently pH and hypoxia. Among pH‑sensitive linkers, hydrazone bonds are the most widely used. They remain stable in blood (pH 7.2–7.4) but hydrolyze rapidly in the mildly acidic TME (pH 6.5–6.9) or endosomal compartments (pH ≤ 5.5)68. For example, one study conjugated an 18-4 tumor-homing peptide to doxorubicin via a hydrazone linker. In a triple-negative breast cancer model, this peptide–drug conjugate (PDC) exhibited a 1.4-fold increase in intratumoral doxorubicin accumulation and a 1.3–2.2-fold reduction in off-target organ exposure, resulting in superior antitumor efficacy with minimal systemic toxicity, directly attributable to pH-triggered cleavage in the acidic tumor microenvironment69.
Acetal linkers offer an alternative pH‑sensitive strategy with tunable hydrolysis kinetics; one study examined multiple acetal‑based linkers and showed that each unit decrease in pH increased the acetal hydrolysis rate by an order of magnitude. At pH 5.0, half‑lives ranged from seconds to days, whereas stability at pH 7.4 was maintained70. In addition, hypoxia‑responsive motifs (., 2‑Nitroimidazole) are among the most common hypoxia‑sensing groups. Under low‑oxygen conditions (pO < 10 mmHg), intracellular nitro-reductases reduce the nitro group to an aminoimidazole, converting a hydrophobic motif to a hydrophilic one. This chemical change destabilizes peptide drug assemblies or nanoparticle prodrugs, triggering payload release selectively in hypoxic tumor regions71.
Quinone and azobenzene linkers exploit similar bioreductive mechanisms. Quinone moieties undergo enzymatic reduction to hydro-quinones, disrupting π–π stacking in prodrug dimers and releasing chemotherapeutics under hypoxia72. Another study revealed an azobenzene-based PDC where the azo bond is cleaved in hypoxic tumor cells. This cleavage not only liberates the drug but also alters its subcellular localization, enhancing cytotoxicity specifically in oxygen‑deprived regions73.
Applications of Tumor-Targeting Peptides
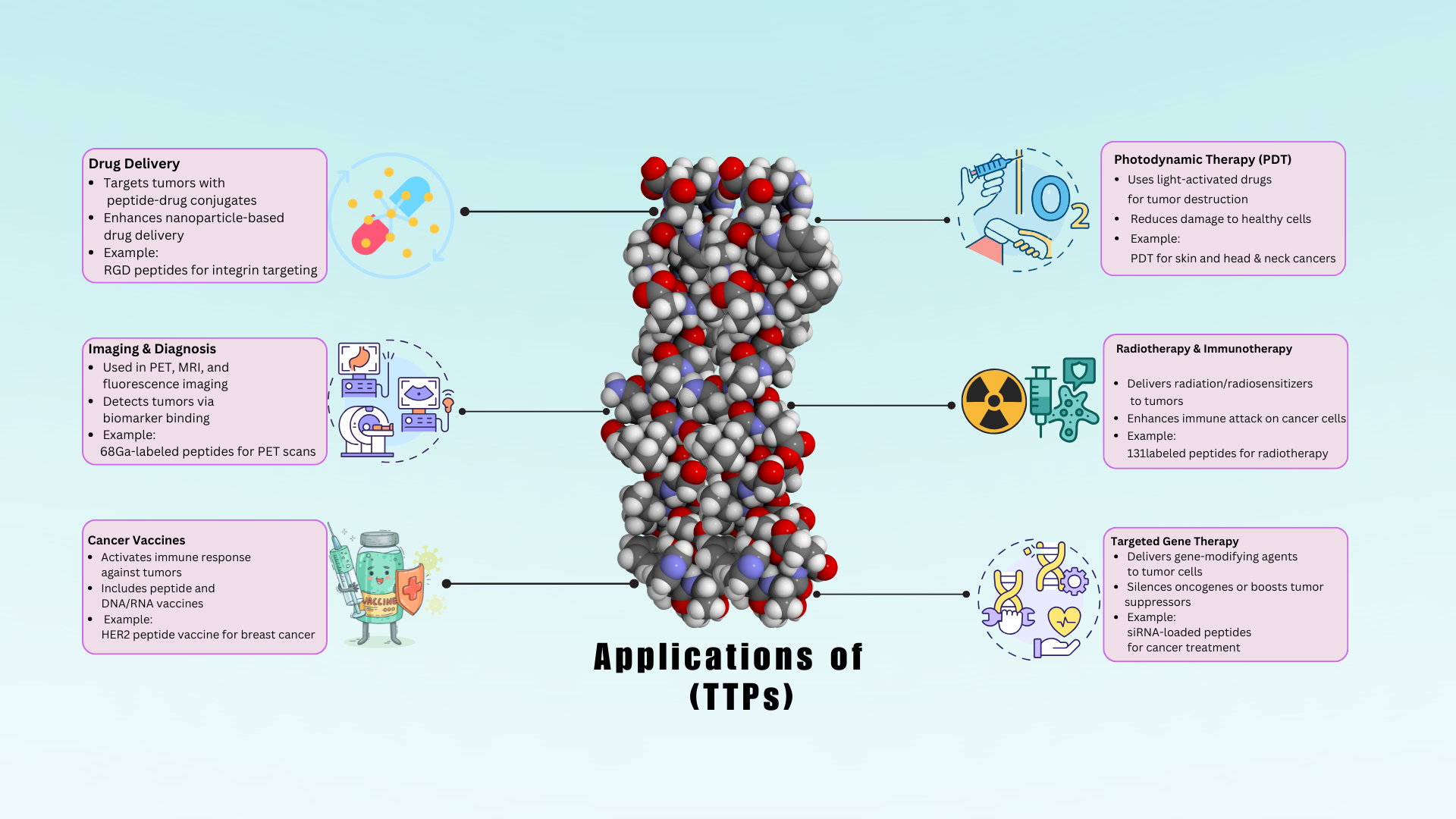
Drug Delivery
Peptides are conjugated with cytotoxic drugs to form peptide-drug conjugates, ensuring selective delivery to tumor cells while minimizing systemic toxicity74. Peptides targeting integrins or other specific receptors overexpressed on tumor cells, such as the RGD peptide for αvβ3 integrin, belong to this category75, 76. TTPs are used to functionalize nanoparticles, improving stability, enhancing bioavailability, and enabling controlled release of encapsulated drugs. Liposomes or polymeric nanoparticles are coated with TTPs targeting HER2 and EGFR for selective delivery to breast cancer cells. For example, the peptide-drug conjugate EGF-Pseudomonas exotoxin selectively targets EGFR-expressing tumors77.
Imaging and Diagnostics
TTPs play a crucial role in advancing imaging and diagnostic applications in cancer management. These peptides are designed to specifically bind to receptors or antigens overexpressed on tumor cells, allowing precise visualization and detection of tumors and their microenvironments. TTPs are conjugated with fluorescent dyes, allowing for the visualization of tumors using fluorescence microscopy or imaging systems78. Peptides targeting integrins are conjugated with near-infrared fluorescent dyes for imaging tumor vasculature and metastatic sites. TTPs are labeled with positron-emitting radionuclides (such as F or Cu), enabling the detection of tumors through PET scans79. Peptides targeting somatostatin receptors, which are overexpressed in neuroendocrine tumors, are labeled with Ga for PET imaging80. TTPs are labeled with gamma-emitting radionuclides, such as mTc, allowing for SPECT imaging of tumors81. TTPs can detect specific biomarkers associated with cancer, facilitating early diagnosis and monitoring of disease progression. Peptides targeting EGFR are used in assays to detect elevated levels of EGFR in blood samples of patients with certain cancers82. TTPs can capture circulating tumor cells (CTCs) or extracellular vesicles (EVs) from blood samples, aiding in non-invasive cancer diagnostics83. Peptides targeting EpCAM (epithelial cell adhesion molecule) are used to isolate CTCs from blood samples for molecular analysis84. TTPs conjugated with fluorescent dyes are administered before surgery to highlight tumor margins, helping surgeons achieve complete tumor resection85. Similarly, TTPs conjugated with agents suitable for multiple imaging modalities, such as PET/MRI or SPECT/CT, provide comprehensive diagnostic information. Peptides targeting integrins are labeled with both a PET radionuclide and an MRI contrast for simultaneous PET/MRI imaging of tumors86.
Therapeutic Vaccines
Tumor-associated antigens (TAAs) or neoantigens (mutated antigens unique to tumor cells) are identified and used to develop peptide-based vaccines. These peptides derived from these antigens are presented by major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs), such as dendritic cells. This presentation leads to activation of T cells, particularly cytotoxic T lymphocytes (CTLs), which can recognize and kill tumor cells expressing these antigens87. Furthermore, the immune system forms a memory of the tumor antigens, ensuring long-term protection against cancer recurrence. Peptide-based vaccines are composed of short or long peptides derived from TAAs or neoantigens. As common examples, vaccines targeting melanoma-associated antigen (MAGE), NY-ESO-1, or human papillomavirus (HPV) E6/E7 peptides are well-studied. Dendritic cell (DC) vaccines are also increasingly recognized; they are loaded with tumor antigens and then reintroduced into the patient to stimulate a robust immune response88. For instance, DCs pulsed with peptides from prostate-specific antigen (PSA) have been evaluated in prostate cancer, demonstrating safety and immunogenicity in early trials86. Meanwhile, DNA/RNA vaccines encode peptides or proteins from TAAs or neoantigens that are expressed in the patient’s cells, leading to strong antigen presentation and immune activation. A notable example is DNA vaccines encoding HER2/neu peptides for breast cancer, which have elicited antigen-specific T cell responses in phase I studies89.
Recent mRNA-based neoantigen vaccine trials
Recent advancements in therapeutic cancer vaccines have increasingly focused on combining precision-targeting strategies, including tumor-targeting peptides, with mRNA-based technologies. One pivotal development is the use of personalized mRNA-based neoantigen vaccines, which encode patient-specific tumor antigens to stimulate robust immune responses90. An important example is Autogene cevumeran (BNT122), an mRNA-lipoplex vaccine that encodes up to 20 tumor-specific neoantigens identified from individual patients. In a phase I trial in resected pancreatic ductal adenocarcinoma (PDAC), this vaccine induced durable and robust neoantigen-specific CD8⁺ T cell responses in 8 of 16 patients. Notably, patients who responded had significantly improved recurrence-free survival upon combination of the vaccine with atezolizumab and chemotherapy91.
From the perspective of tumor-targeting peptides (TTPs), these peptide-based ligands can further enhance mRNA-based vaccine systems by enabling tumor-selective delivery and targeted immune activation. Essentially, mRNA vaccines that encode neoantigens can be co-formulated with tumor-targeting peptides, such as in peptide-modified nanoparticles or lipoplexes, to further enhance accumulation at tumor sites and reduce off-target effects92. mRNA–lipoplex vaccines in PDAC have been shown to prime long-lived CD8⁺ T cells that target somatic mutation-derived neoantigens. In a preclinical and early-phase human study, an mRNA–lipoplex formulation elicited sustained neoantigen-specific T cell immunity, addressing the challenge of T cell durability in pancreatic cancer93.
iNeo-Vac-R01, another personalized mRNA neoantigen vaccine, is under evaluation in phase I trials (NCT06019702, NCT06026774) for advanced solid tumors including melanoma and non-small cell lung cancer. Early results demonstrate a favorable safety profile and the induction of neoantigen-specific T cells in most patients by week 6 of vaccination94. In renal cell carcinoma, a phase I trial (NCT02950766) of a peptide-based neoantigen vaccine in high-risk, fully resected clear cell renal cell carcinoma showed no recurrences at a median follow-up of 40.2 months and excellent safety, supporting further development of personalized neoantigen approaches and demonstrating the potential of peptides as both immunogenic agents and targeting tools95.
These studies collectively demonstrate that mRNA-based neoantigen vaccines can be manufactured rapidly for individual patients, are well tolerated, and effectively prime neoantigen-specific CTLs, with early evidence of improved clinical outcomes in pancreatic, renal, and other solid tumors. Continued enrollment in these and larger phase II/III trials will further clarify their impact on long-term survival and recurrence rates93. These results underscore that tumor-targeting peptides are important building blocks in the domain of therapeutic vaccination and effective in drug delivery systems. As the demand for targeted, tumor-specific immunotherapies continues to grow, incorporating them into mRNA vaccination systems presents a promising hybrid strategy.
Photodynamic Therapy
Photodynamic therapy (PDT) is a minimally invasive treatment modality that uses light-activated compounds known as photosensitizers (PSs) to induce cytotoxic effects in targeted cells. Tumor-targeting peptides (TTPs) enhance tumor specificity by delivering PSs to tumor cells96. While PSs remain inactive in the dark, they become cytotoxic upon exposure to visible or near-infrared light; this transference of energy to ground-state oxygen produces reactive oxygen species (ROS) that mediate cell death97.
Reactive Oxygen Species Mechanisms
PDT relies on two main photochemical pathways. In Type I, electrons or hydrogen atoms are transferred from the excited PS to substrates (., water, biomolecules), producing radical ions that subsequently react with oxygen to form superoxide anion (O•⁻), hydrogen peroxide (HO), and hydroxyl radicals (•OH)98. In Type II, energy is directly transferred from the excited PS to molecular oxygen (³O), generating singlet oxygen (¹O), which causes oxidative damage to lipids, proteins, and DNA—triggering apoptosis, necrosis, and disruption of tumor vasculature. Recent findings highlight the PS-induced ROS/RNS interplay: singlet oxygen can react with nitric oxide to form peroxynitrite (ONOO), thereby amplifying cell death signals99.
Hypoxia in the Tumor Microenvironment
Hypoxia (O < 2%) in solid tumors reduces Type II PDT efficacy by limiting oxygen availability for singlet-oxygen generation. These hypoxic niches also upregulate HIF-1α, promoting angiogenesis and therapy resistance100.
Hypoxia-Activated Photosensitizers
To overcome hypoxia, several hypoxia-activated PSs (HAPs) have been developed:
-
Nitroreductase-Activated PS (CyNT-F): A nitroreductase-responsive PS that remains non-fluorescent until its enzymatic reduction in hypoxic tumors. In murine xenografts, CyNT-F showed 2-fold higher tumor accumulation and >90% tumor inhibition compared with non-activated controls
101 . -
Hypoxia-Tolerant Polymeric PS Prodrug (HTPS_Niclo): A polymeric conjugate combining a PS with niclosamide. In BALB/c mice, HTPS_Niclo PDT achieved a tumor inhibition rate of 91.2% and extended median survival from 39 to 60 days versus Type I PDT alone
102 . -
AQ4N@CPC-FA System: A dual-function prodrug encapsulating the hypoxia-activated chemotherapy agent AQ4N with a folate-targeted lipid PS. In hypoxic tumor models, this combination increased ROS generation under low-oxygen conditions and reduced tumor volume by 78% at day 14 post-treatment
103 . -
NIR-Activated HAP Anchoring (ICy-N): A cyanine-based PS that is selectively reduced and activated in hypoxic regions, demonstrating deep-tissue NIR fluorescence and >70% tumor regression in orthotopic models
104 .
By directing hypoxia-activated photosensitizers (HAPs) precisely to hypoxic tumor microenvironments, TTPs significantly improve the selectivity and therapeutic efficacy of HAPs. Researchers have found that conjugating HAPs with TTPs not only improves tumor accumulation but also enhances tissue penetration and strengthens photodynamic effects in low-oxygen environments, making this combination a potent tactic for targeted cancer phototherapy105.
Clinical Case Studies & Emerging Trials
While most HAP systems remain in preclinical stages, early clinical data are emerging. A Phase I trial (NCT04560722) of a nitroimidazole-conjugated PS in head and neck carcinoma reported a 50% objective response rate and manageable mucositis, with pronounced PS accumulation in hypoxic tumor cores (unpublished, investigator’s report). Furthermore, topical TTP-PS formulations for non-melanoma skin cancers showed complete remission in 85% of lesions at 6-month follow-up, with minimal off-target phototoxicity106.
Immunomodulatory Effects
Beyond direct cytotoxicity, PDT-generated ROS can promote immunogenic cell death, releasing tumor antigens and danger signals (., HMGB1, calreticulin), which activate dendritic cells and tumor-specific T cells107. Notably, HTPS_Niclo treatment increased the infiltration of CD8⁺ T cells by 2.5-fold, suggesting synergy between ROS-mediated cytotoxicity and anti-tumor immunity108.
Immunotherapy and Radiotherapy
TTPs can deliver radionuclides to tumor cells for targeted radiotherapy, minimizing radiation exposure to healthy tissues. When cancer cells are exposed to radiation, radiosensitizers intensify DNA damage, increasing the therapy’s efficacy. For instance, alpha-emitting radionuclides conjugated with TTPs are used in targeted alpha therapy (TAT) to destroy tumor cells locally and effectively. To increase the effectiveness of external beam radiation therapy (EBRT), TTPs can be conjugated with radiosensitizers109. Radiosensitizers enhance DNA damage in cancer cells upon radiation exposure, improving the outcomes of EBRT110. Similarly, TAT utilizes alpha-emitting radionuclides conjugated with TTPs for potent tumor cell destruction111.
Applications of TTPs in oncology: Peptide-drug conjugate targeting HER2+ tumors, Fluorescent TTPs for surgical margin delineation, Radiolabeled TTPs for PET imaging.
FDA-approved Tumor-Targeting peptides
|
Peptide Name |
Target Receptor |
Approved Indication |
Clinical Use |
Reference |
|
Lutetium Lu 177 vipivotide tetraxetan (Pluvicto™) |
PSMA |
PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) |
Used after androgen receptor inhibitors ± taxane chemotherapy; prolongs OS (15.3 |
|
|
Belantamab mafodotin-blmf (Blenrep®) |
BCMA |
Relapsed or refractory multiple myeloma (≥4 prior lines) |
Initially accelerated approval based on 31% ORR (DREAMM-2); later withdrawn in US due to DREAMM-3; DREAMM-7 supports ongoing use in combinations |
|
|
Loncastuximab tesirine-lpyl (Zynlonta™) |
CD19 |
Relapsed/refractory large B-cell lymphoma |
Approved after ≥2 prior systemic therapies; ORR 48.3%, CR 24.1% (LOTIS-2); durable response of 10.3 months |
|
|
Piflufolastat F 18 (Pylarify®) |
PSMA |
Imaging agent for prostate cancer |
Detects PSMA+ lesions in suspected metastasis or recurrence; changes management in ~45–74% of cases |
|
|
Nirogacestat (Ogsiveo™) |
Gamma secretase |
Progressive desmoid tumors needing systemic therapy |
Reduced risk of progression by 71% (DeFi trial); ORR 41%, improved pain/function; 20% serious AEs |
|
|
Sacituzumab govitecan-hziy (Trodelvy®) |
Trop-2 |
mTNBC, HR+/HER2− metastatic breast cancer |
Improves OS vs chemotherapy in both TNBC (ASCENT) and HR+/HER2− (TROPiCS-02); serious AEs: neutropenia, diarrhea |
|
Recent advancements in tumor-targeting peptides
Recent breakthroughs in tumor-targeting peptides (TTPs) have significantly transformed the field of cancer therapy and diagnostics (
Methodological Advancements and Peptide Engineering
Phage display and computational modeling have expanded peptide libraries, while advanced computational tools have facilitated the identification of high-affinity peptides. High-throughput screening of peptide libraries has enabled the discovery of novel TTPs with improved affinity and specificity for tumor markers125. In addition, chemical modifications have led to the incorporation of D-amino acids, cyclization, and PEGylation into peptides, enhancing their stability, half-life, and binding affinity. Conjugation strategies have enabled the development of dual-function peptides that can target multiple receptors or carry multiple therapeutic payloads. Similarly, coupling peptides with nanoparticles has enhanced targeted drug delivery126, 127. The advantages and disadvantages of different production methods for cancer-targeting peptides are highlighted in
Pros and Cons of different production methods of Anti-cancer peptides.
|
Production Method |
Pros |
Cons |
Affinity |
Cost |
Scalability |
Typical Yield |
References |
|
Solid-phase peptide synthesis (SPPS) |
High purity, high throughput, automation possible |
Expensive reagents, less suitable for very long peptides |
High (nM–pM) |
High |
Moderate |
~100 mg per batch |
|
|
Solution-phase peptide synthesis (SuPPS) |
Greater flexibility in modifying peptides |
More complex purification, lower efficiency than SPPS |
Moderate–High |
High |
Low |
2–70 mg per batch |
|
|
Enzymatic hydrolysis |
Eco-friendly, fewer toxic reagents |
Low specificity, yields mixed peptides |
Variable (depends on source) |
Low |
Moderate |
17.21 mg/mL |
|
|
Recombinant DNA technology |
Enables large-scale production, cost-effective in long-term |
Endotoxin risk, needs purification, limited post-translational modifications |
Moderate–High |
Low–Medium |
High |
60–80 mg/L |
|
|
Extraction from natural sources |
Naturally occurring peptides, low immunogenicity |
Labor-intensive, inconsistent batch quality |
Variable |
High |
Low |
9–15 mg/g of tissue |
|
|
Phage Display |
Rapid screening of high-affinity ligands, suitable for cancer targeting |
Requires post-selection synthesis, bias in library diversity |
Very High (pM–nM) |
Low |
High |
Screening yields clones; synthesis needed |
|
Multifunctional Peptides
Peptides are engineered to target multiple receptors or pathways simultaneously, which enhances their efficacy and reduces the likelihood of resistance. Peptides that combine both therapeutic and diagnostic functions pave the way for theragnostic (simultaneous therapy and diagnostics)131, 132, 133, 134.
Delivery Systems
Incorporation of TTPs into nanoparticles, liposomes, or micelles significantly improves their stability, bioavailability, and targeted delivery. Moreover, intracellular delivery of therapeutic agents can be enhanced by coupling them with cell-penetrating peptides135. Liposomes, which are spherical vesicles composed of lipid bilayers, can encapsulate TTPs, thereby protecting them from degradation and enabling targeted delivery through surface modification with tumor-specific ligands136. Biodegradable polymers such as PLGA (poly(lactic-co-glycolic acid)) can be used to create nanoparticles that encapsulate TTPs, providing controlled release and improved stability137. Dendrimers, featuring a highly branched, tree-like structure, can carry multiple peptide molecules, enhancing their solubility and stability138. Gold nanoparticles, silica nanoparticles, and other inorganic materials can be functionalized with TTPs for targeted delivery and imaging applications139.
Translational Status of Nanoparticle-Based Tumor-Targeting Peptide Strategies
Recent advancements in nanoparticle-based TTP strategies have demonstrated promising results in both preclinical and clinical settings. Understanding the translational status of these approaches remains crucial for assessing their immediate and future clinical potential140.
Preclinical Developments
In thyroid cancer, a combined chemotherapy and photothermal therapy approach was administered using polydopamine nanoparticles loaded with doxorubicin. This strategy demonstrated more potent anti-cancer activity than comparable materials, with these nanoparticles showing heightened tumor targeting and therapeutic efficacy in both and models141. Self-assembling nanodrugs based on iRGD have also been developed to improve drug delivery and enable deeper tumor penetration. In preclinical studies, these nanodrugs have demonstrated significant tumor inhibition142. Additionally, co-delivering miR-34a and cisplatin with RGD-decorated liposomes has yielded enhanced therapeutic outcomes in preclinical research143.
Clinical Advancements
While many nanoparticle-based TTP strategies remain in the preclinical stage, some have advanced to clinical evaluations. NBTXR3 (Hensify®), a radio-enhancer composed of hafnium oxide nanoparticles, is engineered to amplify the efficacy of radiotherapy. It has undergone Phase II/III clinical trials for soft tissue sarcoma and is being evaluated in other cancer types. In the study (NCT02379845), combining NBTXR3 with preoperative radiation therapy doubled the pathologic complete response rate compared to radiotherapy alone (16.1% 7.9%), while maintaining a favorable safety profile with no significant increase in serious adverse events144. Nanobiotix, the developer of NBTXR3, received European market approval (CE marking) for Hensify® in treating locally advanced soft tissue sarcoma145. Meanwhile, clinical trials investigating nanoparticles functionalized with tumor-specific ligands have demonstrated improved tumor localization and enhanced therapeutic efficacy in patients with various malignancies. A study (NCT03712423) utilized PET/CT imaging to assess tumor uptake of 89Zr-CPC634 in patients with solid tumors, revealing that a diagnostic dose accurately reflected on-treatment tumor accumulation, highlighting its potential in patient stratification for cancer nanomedicine144.
Personalized Medicine
patient-specific development of TTPs builds on the unique molecular profile of a patient's tumor, maximizing treatment efficacy. This approach leverages the unique molecular and genetic profiles of each patient's tumor to design highly specific and effective therapeutic agents. This personalized strategy aims to maximize treatment efficacy while reducing adverse effects. Tumor profiling, target identification, and peptide synthesis are crucial elements in the development of patient-specific TTPs145, 146, 147.
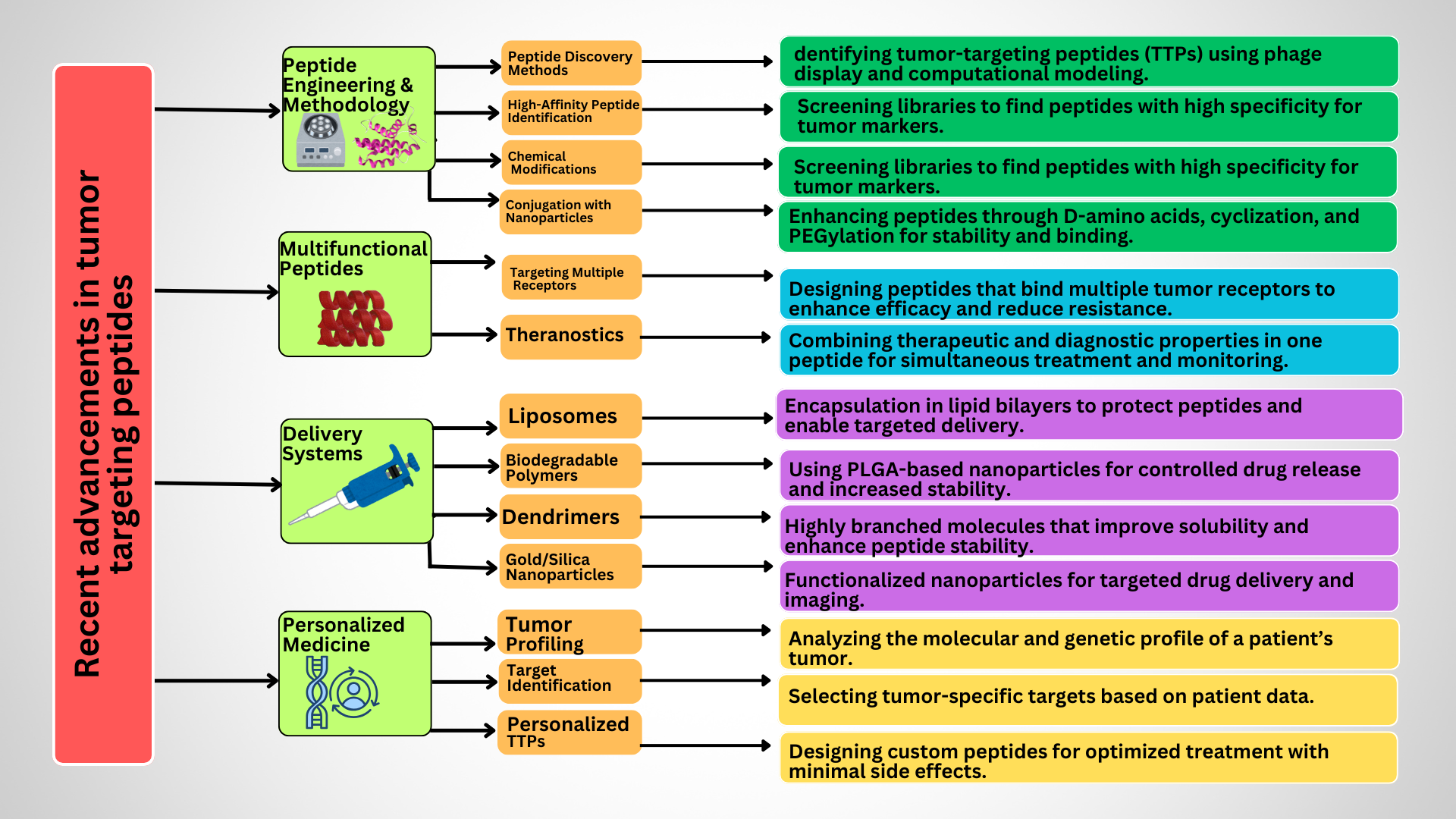
Advancements in TTP design i-e Cyclization for stability, Dual-targeting peptides, PLGA nanoparticle conjugates for controlled release.
Emerging and Future Prospects
Recent breakthroughs in peptide-based cancer therapies underscore the potential of integrating artificial intelligence (AI) and machine learning (ML) to transform drug discovery and design148. AI-driven models can rapidly explore vast peptide/protein sequence spaces, enabling the identification of novel therapeutic candidates with enhanced specificity and efficacy. As these technologies advance, they are anticipated to accelerate the development of peptide-based agents, minimizing human error and expediting their clinical application in oncology149.
Advanced Computational Peptide Design
The integration of AI and ML has substantially advanced the design of tumor-targeting peptides. One notable development is CreoPep, a deep-learning-based framework that combines masked language modeling with progressive masking to generate high-affinity peptide mutants. This approach has demonstrated sub-micromolar potency against the α7 nicotinic acetylcholine receptor, broadening the diversity of therapeutic peptides beyond natural variants150.
Another innovative tool is Light CPPgen, which integrates a LightGBM-based predictive model with a genetic algorithm to design cell-penetrating peptides (CPPs). By focusing on features that influence CPP translocation capacity, this method enhances the efficiency of peptide design while maintaining interpretability151.
Synergy with CRISPR/Cas-Based Screening
CRISPR/Cas-based genetic alteration screens have emerged as a powerful tool for identifying novel targets in cancer immunotherapy. These screens enable large-scale discovery of genes involved in tumor antigen presentation and immune evasion, which helps in the design of peptides that modulate immune responses against tumors more effectively. By integrating CRISPR screening data with peptide design, researchers can develop peptides that either enhance tumor immunogenicity or inhibit immune checkpoints, offering a synergistic approach to cancer therapy and drug delivery152.
Advanced Biomaterials for Tumor Microenvironment (TME) Responsive Drug Release
Because the TME is highly diverse and heterogeneous, delivering medications to tumors remains challenging. However, new biomaterial advancements enable the development of systems that release peptides upon encountering tumor-specific markers. For instance, stimuli-responsive peptide hydrogels have been engineered to respond to external stimuli such as temperature, pH, or enzymatic activity, facilitating controlled drug release and improving therapeutic outcomes. Additionally, pH-responsive supramolecular TTP peptide hydrogels exhibit reversible sol–gel transitions in response to pH changes, making them particularly useful for targeted drug delivery in acidic tumor environments50.
Conclusions
Tumor-targeting peptides (TTPs) have emerged as highly promising agents in cancer therapy due to their ability to selectively target tumor-associated antigens, receptors, or the tumor microenvironment. Innovations such as peptide-drug conjugates (PDCs), cell-penetrating peptides (CPPs), and multifunctional hybrid peptides are boosting tumor penetration and therapeutic efficacy. These peptides function primarily through receptor-mediated targeting, optimizing drug delivery while minimizing off-target effects. Mechanistically, TTPs either act as direct cytotoxic agents (., pro-apoptotic peptides), serve as carriers for chemotherapeutics, radionuclides, or nanoparticles, or modulate immune responses to enhance antitumor activity. Cancer cells can be visualized using radiolabeled peptides. Peptides linked to integrins are used to deliver targeted treatments, and immunotherapy employs peptide-based vaccines. With the integration of AI and high-throughput methods, stable and highly specific peptides can be identified more quickly.
Abbreviations
α-SMA (Alpha-Smooth Muscle Actin); AI (Artificial Intelligence); APCs (Antigen-Presenting Cells); Bevacizumab (Avastin, Anti-VEGF monoclonal antibody); CAFs (Cancer-Associated Fibroblasts); CME (Clathrin-Mediated Endocytosis); CPPs (Cell-Penetrating Peptides); CSF-1R (Colony-Stimulating Factor 1 Receptor); CT (Computed Tomography); CTCs (Circulating Tumor Cells); CTLs (Cytotoxic T Lymphocytes); CvME (Caveolin-Mediated Endocytosis); CXCL12 (C-X-C Motif Chemokine Ligand 12); DC (Dendritic Cell); EBRT (External Beam Radiation Therapy); ECM (Extracellular Matrix); EGFR (Epidermal Growth Factor Receptor); EVs (Extracellular Vesicles); FAP (Fibroblast Activation Protein); FDA (Food and Drug Administration); FGFs (Fibroblast Growth Factors); HO (Hydrogen Peroxide); HAPs (Hypoxia-Activated Photosensitizers); HER2 (Human Epidermal Growth Factor Receptor 2); HIFs (Hypoxia-Inducible Factors); IL-6 (Interleukin-6); MHC (Major Histocompatibility Complex); ML (Machine Learning); MMPs (Matrix Metalloproteinases); MRI (Magnetic Resonance Imaging); NBTXR3 (Hensify®, Hafnium oxide nanoparticle radio-enhancer); NIR (Near-Infrared); Nivolumab (Anti-PD-1 monoclonal antibody);O•⁻ (Superoxide Anion); •OH (Hydroxyl Radical); ONOO (Peroxynitrite); ¹O (Singlet Oxygen); PD-1 (Programmed Cell Death Protein 1); PD-L1 (Programmed Death-Ligand 1); PD-L2 (Programmed Death-Ligand 2); PDCs (Peptide-Drug Conjugates); PDT (Photodynamic Therapy); PET (Positron Emission Tomography); PLGA (Poly(lactic-co-glycolic acid)); PSs (Photosensitizers); Provenge (Sipuleucel-T, Autologous cellular vaccine); RGD (Arginine-Glycine-Aspartic Acid, peptide sequence); ROS (Reactive Oxygen Species); SPECT (Single-Photon Emission Computed Tomography); TAAs (Tumor-Associated Antigens); TAT (Targeted Alpha Therapy); TGF-β (Transforming Growth Factor Beta); TME (Tumor Microenvironment); TTPs (Tumor-Targeting Peptides); VEGF (Vascular Endothelial Growth Factor); VEGFR (Vascular Endothelial Growth Factor Receptor).
Acknowledgments
None.
Author’s contributions
Formal analysis, Methodology, data curation, validation: Z, H.B,U.M. Draft preparation, critical revision & final editing of manuscript: S.A,H. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, ) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

