Comparison of injection of midazolam-fentanyl with pethidine in management of pain induced by extracorporeal shock wave lithitripsy: A randomized clinical trial
- Nursing Department, Faculty of Nursing, North Khorasan University of Medical Sciences, Bojnurd, Iran
- MD, Department of Urology, Imam Hasan Hospital, North Khorasan University of Medical Sciences, Bojnurd, Iran
- Instructor of Medical Surgical Nursing, Department of Nursing, Esfarayen Faculty of Medical Sciences, Esfarayen, Iran
- Nursing Department, Shirvan Center of Higher Health Education, North Khorasan University of Medical Sciences, Bojnurd, Iran
Abstract
Introduction: Urinary stones are the third most common disease of the genitourinary tract after urinary tract infections and prostate diseases. One of the ways to remove a urinary stone is extracorporeal shock wave lithitripsy, which crushes the stones for easier removal, and it is often used to reduce pain, reduce anxiety, and stabilize the patient. In this regard, the use of effective analgesics with less serious side effects seems reasonable.
Methods: This randomized clinical trial study was performed with 90 patients who were divided into two groups according to a random number table. The first group received pethidine, and the second group received midazolam and fentanyl (midazolam-fentanyl). The type of medication used and demographic information were recorded, and the patients’ pain was assessed by a visual analog pain scale at 15, 30, 45, and 60 minutes.
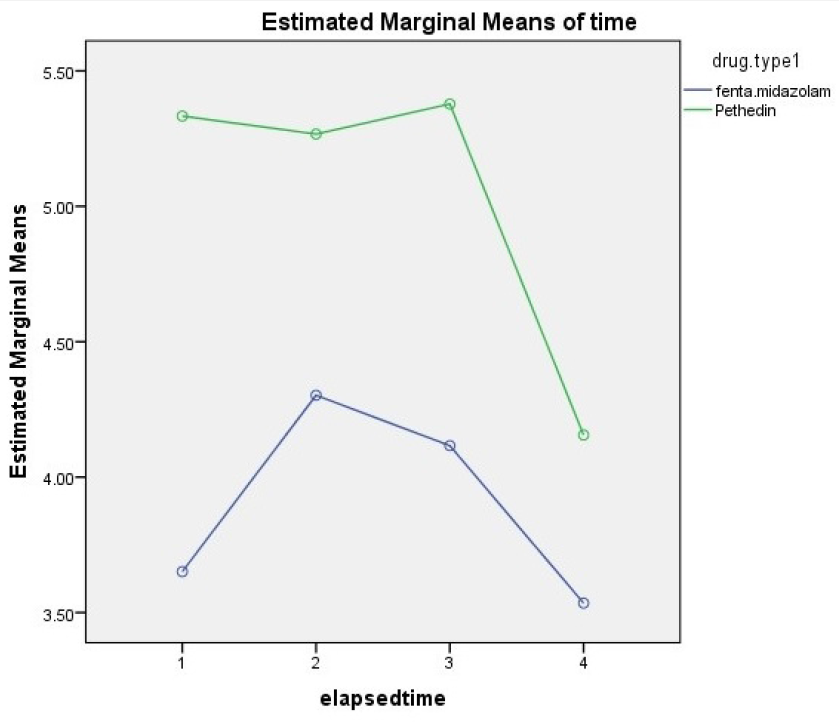
Results: Of the subjects, 59 (65.5%) were male and 30 (33.5%) were female. The mean age of the patients was 38.4 ± 13.5 years. The level of pain at 15 minutes was 3.71 ± 2.4 in the midazolam-fentanyl group and 5.33 ± 2.9 in the pethidine group. At 45 minutes, the pain level was 4.2 ± 3.1 in the midazolam-fentanyl group and 5.26 ± 2.72 in the pethidine group. The differences between groups was significant at 15 and 45 minutes. At 30 and 60 minutes, the pain was lower in the midazolam-fentanyl group than in the pethidine group, but these differences were not statistically significant. There was no significant difference between the two groups with respect to the incidence of nausea and vomiting, restlessness, and anxiety.
Conclusion: This study showed that the pain reported by patients using fentanyl-midazolam was lower than the pain reported by patients on pethidine, and the pain decreased with time in both groups. Therefore, if there is no other indication for the use of drugs, the combination of fentanyl-midazolam will have a better effect on pain and should be used.
Trial registration: Current Controlled Trials IRCT2016051427893N1.
Introduction
Urinary stones are the third most common disease of the genital tract after urinary tract infections and prostate diseases1. The prevalence of these stones varies by age, sex, race, and geographic region. Its prevalence has been reported to be 1-15% throughout a typical lifetime2. Stone removal is performed in several ways3; one of the most commonly used semi-invasive stone removal methods is extracorporeal shock wave lithotripsy to crush the stones. This is the treatment of choice in most urinary stones less than 20 mm wide4, 5, 6.
One of the most common complications of stone crushing is pain. Despite new technologies that have reduced pain, various medications have been used often to relieve pain, reduce anxiety, and reduce patient mobility during the procedure. Each of these medications has different advantages and disadvantages. Patients should have a short period of awakening after the procedure, and the use of anesthetics and long-acting analgesics should be limited. Therefore, the use of effective analgesics with fewer side effects and less drowsiness seems reasonable7. Some studies have not directly recommended the use of potent drugs, such as remifentanil, because of its respiratory effects8. Other studies have suggested using anesthetics, such as ketamine, that have lower respiratory effects at low doses9. Some studies have evaluated drugs like opioids, anesthetics, and non-steroidal anti-inflammatory drugs10, 11. Despite this technique being widely used, it has no specific standard protocol for pain control, and opinions on the methods of pain medication and techniques for this operation vary widely3. Pain can cause the patient to move during stone crushing and disrupt the procedure. Reducing pain is important because pain can disrupt one’s energy, affect one’s ability to communicate and interact socially, and skew one’s understanding of the meaning of life. The need to identify an effective way and suggest a more effective medication for pain management has motivated us to take steps to address this lack of a protocol. We conducted a study to compare the effects of pethidine and midazolam with general anesthesia on pain relief during extramedullary crushing.
Materials - Methods
This randomized clinical trial study was performed with 90 patients referred to the North Khorasan University of Medical Sciences (Iran) for lithotripsy. All patients were monitored for long-term opiate use, continuous analgesic use, and history of psychiatric disorders. Inclusion criteria included consent to participate in the research, reading and writing skills, being alert at the right level of communication, no long-term history of taking opioids and painkillers continuously, no history of mental disorders, high risk of anesthesia according to ASA charts (score of 1 or 2), and a history of allergic reactions to drugs (especially anesthesia drugs). Exclusion criteria included lack of consent to cooperate at any time, patients who were unable to work with the researcher (due to unconsciousness, severe hearing and speech problems, and inability to communicate), patients who for whatever reason required deeper anesthesia and other medications following lithotripsy, and patients who were contraindicated for opiate drugs or anesthetics (Figure 1 ).

Consort flow diagram of the study.
If the patient needed a non-prescription medication during the operation, it was administered but then the patient was excluded from the study, and another patient was selected to replace him or her. The patients were divided into two groups of 45 with a random numbers table. The first group received 0.5 mg/kg pethidine (produced by Hameln) after the first injection, and the second group received midazolam-fentanyl [midazolam (produced by Daroo Pakhsh) at 1to 2 mg dose and fentanyl (produced by Daroo Pakhsh) at 1 to 2 μg/kg dose]. An anesthesiologist performed the injections. All patients underwent expert anesthesia and standard monitoring throughout the duration of the lithotripsy. The lithotripsy was performed for all participants, and the voltage, time of lithotripsy, and frequency of exposure to the ultrasonic waves were recorded. The type of medication used and demographic information were also recorded in the relevant checklists, as well as patients’ pain levels as assessed by a visual analog pain scale (VAS) at 15, 30, 45, and 60 minutes.
Ethical considerations
This study was approved by the Medical Research and Ethical Committee of Esfarayen University of Medical Sciences (IR.ESFARAYENUMS.REC.1394.11).
Data analysis
The completed patient checklists were coded and entered into SPSS 20 without anonymity. The study was blind in that pain assessors and statistical analyzers did not know the type of drug used or the group to which the patient belonged. An independent t-test was used to compare the pain in the two groups – midazolam-fentanyl and pethidine – and to compare the differences between the nominal factors and the chi-square test.
Demographic characteristics and comparisons between the two groups
| Variable | Group 1 (Fentanyl-midazolam) | Group 2 (Pethidine) | P-value |
|---|---|---|---|
| Age | 43.07 ± 41.14 | 32.42 ± 35.12 | 0.042 |
| Size of stone in millimeters | 30.20 ± 11.3 | 98.90 ± 10.30 | 0.67 |
| The number of shock waves given | 36.80 ± 3561.66 | 44.17 ± 3544.44 | 0.89 |
| Voltage used | 27.90 ± 72.30 | 12.11 ± 73.50 | 0.38 |
VAS pain scores and comparison between groups at different times
| Variable | Group 1(Fentanyl-midazolam) | Group 2 (Pethidine) | P-value |
|---|---|---|---|
| Pain at 15 minutes | 2.42 ± 3.71 | 2.96 ± 5.33 | 0.006 |
| Pain at 30 minutes | 3.10 ± 4.33 | 2.72 ± 5.26 | 0.13 |
| Pain at 45 minutes | 2.96 ± 4.2 | 2.59 ± 5.37 | 0.048 |
| Pain at 60 minutes | 3.11 ± 3.53 | 2.24 ± 4.15 | 0.36 |
Results
In this study, 90 patients were divided into two groups (n = 45 per group). Of the subjects, 59 (65.5%) were male and 30 (33.5%) were female. The mean age of the patients was 38.4 ± 13.5 years. The mean stone size was 11.14 ± 3.5 mm. Of the subjects, 74 (82.2%) had no history of the disease, 5 (5.6%) had hypertension, and 6 (6.7%) had diabetes (
The pain level measured by the VAS in the midazolam-fentanyl group was 3.71 ± 2.4 and in the pethidine group was 5.33 ± 2.9 at 15 minutes post-operation. The pain level reported by the midazolam-fentanyl group was significantly lower (p=0.006). At 30 minutes, the mean pain was 4.33 ± 3.10 in the midazolam-fentanyl group and 5.11 ± 2.72 in the pethidine group. This difference in mean pain was not significant, despite the decrease found in the midazolam-fentanyl group. The level of pain on the VAS at 45 minutes after lithotripsy was 4.2 ± 3.1 in the midazolam-fentanyl group and 5.26 ± 2.72 in the pethidine group (pain was significantly higher in the pethidine group). At 60 minutes, the mean pain score in the midazolam-fentanyl group was significantly lower than that of the pethidine group, but this difference was not statistically significant (

The amount of pain changes over time.
Comparison of the incidence of postoperative complications in the two groups
| Type of complication | Pethidine | Fentanyl-midazolam | P-value | ||
|---|---|---|---|---|---|
| No | Yes | No | Yes | ||
| Nausea | 40 | 5 | 9 | 5 | 0.97 |
| Restlessness | 43 | 0 | 43 | 1 | 0.32 |
| Vertigo | 30 | 15 | 21 | 24 | 0.056 |
Discussion
Extracorporeal shock wave lithotripsy is one of the most common operations for breaking and removing urinary stones. Pain relief seems necessary, due to the patient’s discomfort during stone crushing and inability to move, but anesthesia is not common for this purpose. Therefore, injectable, topical, and other painkillers are recommended. Medication with opiates is usually preferred because they guarantee patient comfort with easy recovery and fewer side effects12. Therefore, finding a suitable way to reduce pain can help patients and therapists. Pethidine and midazolam-fentanyl were used to reduce pain in both groups. The mean pain reduction at 15 and 45 minutes in the midazolam-fentanyl group was significantly different from that in the pethidine group. A VAS was used for pain measurement, and the results showed that the pain intensity in the midazolam-fentanyl group was less than that in the pethidine group at 20 minutes and 2 hours, but this difference was not statistically significant4.
In our study, there was a decrease in pain severity over time, but the difference between the two groups was significant only at 45 and 15 minutes. However, it is noted that although Mehrabi used opiate analgesics, their drug combination was a little different from ours. In a 2013 study conducted by Lee at the Center for Urology in China, three drugs were assessed for pain relief from crushing shock: a sodium diclofenac analgesic, a eutectic mixture of local anesthetic, and a diclofenac gel. The organs were compared in the study and there were no significant differences found among the three drugs13. Batch published a review article entitled "Drugs for pain management in shock wave lithotripsy". In that study, drug use was introduced as one of the best ways to control pain in patients using shock wave lithotripsy3. This is in line with the reduction of pain in the two groups in our study. A study by Alibeigi compared the effects of piroxicam and pethidine2. In a study by Ezkan , midazolam, fentanyl, diclofenac, and tramadol were used for pain management after extracorporeal shock wave lithotripsy; their study found a preference for diclofenac and tramadol over midazolam and fentanyl14. Our study used two types of opioids. In their study of nausea, Demir made a comparison of pethidine and diazepam with diclofenac and hyoscine, and found that pethidine and diazepam had a greater effect on pain relief than did diclofenac and hyoscine. With respect to vomiting, their study did not observe a difference between the two groups. Likewise, in our study, nausea and vomiting (and other complications like dizziness and restlessness) were not significantly different between the two groups. Moreover, Demir used pethidine and compared it to a non-opioid drug; there were no significant differences also in their study with respect to side effects like nausea and vomiting15.
The main limitations of our study were the confined location of the study and the prolonged sampling procedure.
Conclusion
This study showed that the lithotripsy pain reported by patients using fentanyl-midazolam was lower than that reported by patients using pethidine, and that pain decreased with time in both patient groups. Therefore, if there is no other indication for the use of drugs, the combination of fentanyl and midazolam will probably have a better effect on pain, and should be considered for pain management.
Abbreviations
ESWL: Extracorporeal shock wave lithotripsy
Acknowledgments
This article was derived from a research project approved by the Esfarayen Faculty of Medical Sciences, which was conducted with the financial support of this university. The researchers would like to express their gratitude to the Faculty Research Council and all patients, who participated in this study.
Author’s contributions
M.Z., A.K. and H.M. contributed to the conceptualization and design of the study, the acquisition, analysis and interpretation of data. M.Z. and R.Z. were for drafting the article and revising the article critically for important intellectual content. All authors read and approved the final manuscript.
Funding
This article was derived from a research project approved by the Esfarayen Faculty of Medical Sciences, which was conducted with the financial support of this faculty.
Availability of data and materials
Data and materials used and/or analysed during the current study are available from the corresponding author on reasionable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board (Medical Research and Ethical Committee of Esfarayen Faculty of Medical Sciences) approved the study (IR.ESFARAYENUMS.REC.1394.11), and all participants provided written informed consent.
Consent for publication
The authors hereby consents that the Publisher publishes the Work.
Competing interests
The authors declare that they have no competing interests.

