Association of anti-BCOADC-E2 autoantibodies with increased bilirubin levels in Chinese primary biliary cholangitis patients
- Nanjing University, China
Abstract
Introduction: The clinical significance and prevalence of anti-mitochondrial antibodies (AMA) other than PDC-E2 have not yet been thoroughly investigated in Chinese patients with primary biliary cholangitis (PBC). This study aims to investigate the overall prevalence and clinical significance of different AMAs in Chinese PBC patients.
Methods: Enzyme-linked immunosorbent assays were developed using purified recombinant pyruvate dehydrogenase complex-E2 (PDC-E2), branched-chain 2-oxo-acid dehydrogenase complex (BCOADC-E2), and 2-oxo-glutaric acid dehydrogenase complex (OGDC-E2) proteins. Serum samples from 1,096 PBC patients were tested for antibody detection. AMA titers were measured in a subset of ten PBC patients both before and after ursodeoxycholic acid (UDCA) treatment. Statistical analyses were performed using antibody results and biochemical data from these PBC patients. Results: The overall prevalence of AMAs against PDC-E2, BCOADC-E2, and OGDC-E2 was 86.13%, 84.21%, and 40.70%, respectively, among PBC patients. The prevalence of anti-BCOADC-E2 was much higher in Chinese patients compared with Caucasian PBC patients. The presence of anti-BCOADC-E2 autoantibodies was significantly associated with elevated bilirubin concentration (P < 0.001). A significant decrease in anti-BCOADC-E2 titers was observed in half (5/10) of the PBC patients after UDCA treatment; among these patients, two became negative after 15–30 months of UDCA treatment.
Conclusions: In conclusion, our results suggest that anti-BCOADC-E2, which may be a serological marker for the early diagnosis of PBC, is the only AMA that is significantly affected by UDCA treatment.
Introduction
Primary biliary cholangitis (PBC) is a chronic autoimmune genetic disorder characterized by the occurrence of serum anti-mitochondrial antibodies (AMA), lymphocytic infiltration of the portal tract, progressive intrahepatic destruction of the interlobular bile ducts, which eventually leads to cirrhosis of the liver1. An increasing prevalence of PBC has been observed worldwide, presumably due to improved diagnosis, better care and survival of PBC patients, as well as enhanced awareness and knowledge of the disorder among clinicians2. The etiology and pathogenesis of PBC are poorly understood. It is possible that exposure to environmental factors triggers the disease process in genetically susceptible individuals3.
AMA, a disease-specific autoantibody found in 90% of PBC patients, is the characteristic serological hallmark of PBC4. The target antigens are members of the 2-oxo-acid dehydrogenase complex (2-OADC), including pyruvate dehydrogenase complex-E2 (PDC-E2), the branched chain 2-oxo-acid dehydrogenase complex (BCOADC-E2), and the 2-oxo-glutaric acid dehydrogenase complex (OGDC-E2). Each of the 2-OADC members has distinct antigenicity and shows no cross-reactivity5. The dominant epitopes contain a lysine-lipoyl acid domain, that can be recognized by both B and T cells and is subject to xenobiotic modifications6. It is unknown whether the presence of AMAs targeted to different antigens is predictive or prognostic for a particular clinical and biological profile of PBC. The clinical significance and prevalence of AMAs other than PDC-E2 have not been analyzed among PBC patients in the Chinese population.
In this study, we examined the prevalence and clinical significance of three different AMAs using enzyme-linked immunosorbent assay (ELISA) and investigated the association between these autoantibodies and the clinical and biochemical features in a large cohort of Chinese PBC patients.
Methods
Subjects
This study was conducted on patient samples collected from the member hospitals of the Jiangsu Provincial PBC Collaboration Group (JSPPCG) as part of a genome-wide association study7. PBC patients were recruited according to the guidelines of the Declaration of Helsinki (2008). The diagnosis of PBC was based on the following criteria: AMA positive (PDC-E2, BCOADC-E2, or OGDC-E2); AMA negative, anti-sp100 or -gp210 positive, with elevated alkaline phosphatase; or antibody negative, but with histological evidence and elevated alkaline phosphatase. Since a liver biopsy was not conducted for most patients, those who tested negative for AMA, sp100, and gp210 antibodies and did not undergo biopsy were excluded.
Biochemical test records for 780 of the 1096 PBC patients in this study were included at the time of disease onset (Supplementary table 1). Of these 780 patients, 666 were female (85.38%) and 114 were male (14.62%). The median age of the patients was 55.9 years. Patients who had received ursodeoxycholic acid (UDCA) at a daily dose of 13 to 15 mg/kg of body weight and had at least 1 year of follow-up were selected to evaluate their response to UDCA. The biochemical response to treatment was evaluated according to the Barcelona (BA) and Paris I (PA) criteria8.
Cloning and expression of PDC-E2, BCOADC-E2 and OGDC-E2
For plasmid construction, homologous recombination/ET cloning was used to insert gene into the expression vectors9. pET28a with 6×His tag was used to clone human with the following primers (PDC-E2. FP: 5’-CAGCCATCATCATCATCATCA CGGATCCAGTCTTCCCCCGCATCAGAA, PDC-E2.RP: 5’-TGCTCGAGTGCGGCCGCAA GCTTCATTACAACAACATAGTGATAG; BCOADC-E2. FP: 5’-CATCATCATCATCATCACGGAT CCGGACAGGTTGTTCAGTTCAA-3’; BCOADC-E2.RP: 5’-GTGGTGGTGGTGGTGCTCG AGTTCAATCTAGTAGCATAAAAGCTGG-3’; OGDC-E2. FP: 5’-ACTTTAAGAAGGAGATATA CCATGGATGACTTGGTTACAGTC-3’; OGDC-E2.RP: 5’-GTGGTGGTGGTGGTGGTGCTC GAGAAGATCCAGGAGGAGGACTCTG-3’).
RNA from human liver cells (HepG2) served as the template for cDNA preparation by reverse transcribed-polymerase chain reaction (RT-PCR) using the oligos (dT) primer. PCR was performed using the high-fidelity LA Taq polymerase (TaKaRa, China). The DNA sequence of the constructed plasmid was verified by DNA sequencing analysis. Plasmids were transformed into BL21 competent cells. The transformed cells were grown at 37°C overnight in Luria-Bertani medium containing 50 µg/mL ampicillin and induced with 1mM isopropylthiogalactoside (IPTG) overnight at 25°C. PDC-E2 was purified from under native conditions by Ni-NTA agarose (QIAGEN, Germany) with elution buffer (50 mM NaHPO, 300 mM NaCl, 250 mM imidazole, pH 8.0). The eluted proteins were loaded to the HiLoad 26/600 Superdex 75 PG column (GE Healthcare Bio-Sciences AB, Sweden) for the final purification of PDC-E2, BCOADC-E2 and OGDC-E2.
Enzyme-linked immunosorbent assay (ELISA) for autoantibody analysis
Gel-purified proteins (antigens) (1 µg/mL) were suspended in 50 mM carbonate buffer (pH 9.6) and coated onto microtiter plates (Thermo-Fisher, USA) overnight at 4°C for enzyme-linked immunosorbent assay (ELISA). After blocking with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), the plates were incubated with sera (1:2000 dilution) for 2 hours at room temperature and then washed five times with PBS containing 0.1% Tween-20 (PBST). Peroxidase-conjugated anti-human immunoglobulin (IgG, IgM, and IgA) antibody (Millipore, Temecula, USA) was added to the plates (1:30,000 dilution, 100 µL/well) and then incubated for 2 hours at room temperature. After washing six times with PBST, 3,3′,5,5′-tetramethylbenzidine (TMB) was added as the substrate, and 2 M sulfuric acid was added as the stop solution. The optical density (OD) was measured using an ELISA plate reader (Bio-Rad, USA) at 450 nm. Reactivity against AMAs was also assessed using commercially available ELISA kits (Shanghai Kexin Biotech Co., Shanghai, China).
Statistical analysis
All statistical analyses were performed using SPSS version 19.0 for Windows (SPSS, Inc., Chicago, IL). Values were expressed as the mean ± SD. A P value less than 0.05 was considered statistically significant for all tests. The Mann-Whitney U-test was used to compare quantitative variables between two groups, and the χ² test was used to compare categorical variables.
Distribution of AMA to the 2-OADC components in Chinese PBC patient
|
|
All Patients (n=1096) | |
|
Antigens |
Positive Patients |
Prevalence |
|
PDC-E2+ |
944 |
86.13% |
|
BCOADC-E2+ |
923 |
84.21% |
|
OGDC-E2+ |
446 |
40.70% |
|
PDC-E2+/BCOADC-E2+/OGDC-E2+ |
372 |
33.94% |
|
PDC-E2 + /BCOADC-E2+ |
793 |
72.35% |
|
PDC-E2+/OGDC-E2+ |
405 |
36.95% |
|
BCOADC-E2+/OGDC-E2+ |
406 |
37.04% |
|
PDC-E2+ alone |
118 |
10.77% |
|
BCOADC-E2 + alone |
96 |
8.76% |
|
OGDC-E2+ alone |
7 |
0.64% |
|
At least 1 of them + |
1081 |
98.63% |
|
All negative |
15 |
1.37% |
Results
Higher Prevalence of AMA to BCOADC-E2 in Chinese PBC Patients
The overall prevalence of AMA to PDC-E2, BCOADC-E2, and OGDC-E2 is summarized in
Biochemical characteristic of PBC patients according to the presence of M2-AMA
|
Clinical Features |
Anti-PDC-E2 |
Anti-BCOADC-E2 |
Anti-OGDC-E2 | ||||||
|
Positive (673) |
Negative (107) |
P value |
Positive (657) |
Negative (123) |
P value |
Positive (316) |
Negative (464) |
P value | |
|
Age |
56.0 ± 11.5 |
55.4 ± 10.4 |
0.461 |
56.0 ± 11.1 |
55.0 ± 12.7 |
0.484 |
56.5 ± 11.3 |
55.5 ± 11.3 |
0.286 |
|
Sex (F/M) |
571/102 |
95/12 |
0.284 |
554/103 |
112/11 |
0.052 |
262/54 |
404/60 |
0.107 |
|
ALT (IU/L) |
121.6 ± 217.1 |
140.4 ± 165.5 |
0.039 | 120. |
145.9 ± 207.2 |
0.172 |
105.2 ± 128.2 |
137.1 ± 251.4 |
0.156 |
|
AST (IU/L) |
120.0 ± 184.0 |
123.2 ± 144.0 |
0.890 |
114.9 ± 152.4 |
150.1 ± 280.6 |
0.795 |
110.3 ± 112.6 |
127.3 ± 212.6 |
0.891 |
|
GGT (U/L) |
357.0 ± 308.7 |
387.0 ± 317.6 |
0.443 |
370.0 ± 314.4 |
312.5 ± 280.5 |
0.022 |
369.7 ± 337.5 |
355.2 ± 289.9 |
0.799 |
|
ALP (U/L) |
339.1 ± 223.4 |
319.0 ± 197.4 |
0.285 |
343.9 ± 221.5 |
296.2 ± 208.0 |
0.003* |
347.5 ± 231.6 |
328.8 ± 211.7 |
0.220 |
|
TB (μmol/) |
44.6 ± 591 |
50.5 ± 94.5 |
0.234 |
47.1 ± 66.8 |
36.2 ± 54.2 |
<0.001* |
42.5 ± 57.8 |
47.3 ± 69.6 |
0.919 |
|
DB (μmol/) |
28.5 ± 45.9 |
32.1 ± 75.7 |
0.072 |
30.5 ± 52.9 |
20.8 ± 38.0 |
<0.001* |
27.3 ± 46.2 |
30.2 ± 54.0 |
0.835 |
|
TP (g/L) |
73.7 ± 9.7 |
73.4 ± 12.3 |
0.760 |
73.7 ± 10.3 |
73.5 ± 8.7 |
0.634 |
74.7 ± 9.7 |
72.9 ± 10.3 |
0.007* |
|
ALB (g/L) |
37.7 ± 7.3 |
39.4 ± 6.6 |
0.018 |
37.8 ± 7.2 |
38.7 ± 6.8 |
0.223 |
37.6 ± 7.6 |
38.1 ± 6.9 |
0.149 |
Increased bilirubin concentration is associated with anti-BCOADC-E2
The biochemical results at disease onset were summarized according to the reactivity of various AMAs (
Characterization of PBC patients used in time course analysis
|
Patients ID |
Gender |
Age at disease onset (years) |
Age at treatment (years) |
Period of treatment (Months) |
PDC-E2 |
BCOADC-E2 |
OGDC-E2 | |||
|
Before |
After |
Before |
After |
Before |
After | |||||
|
Patient 1 |
Male |
46 |
51 |
20 |
>1:16,000 |
1:2,000 |
>1:16,000 |
- |
1:4,000 |
1:500 |
|
Patient 2 |
Female |
68 |
71 |
30 |
- |
- |
1:16,000 |
1:1,000 |
- |
- |
|
Patient 3 |
Female |
42 |
43 |
23 |
1:16,000 |
1:8,000 |
>1:8,000 |
- |
- |
- |
|
Patient 4 |
Female |
46 |
47 |
26 |
>1:8,000 |
>1:8,000 |
>1:8,000 |
1:4,000 |
1:2,000 |
1:2,000 |
|
Patient 5 |
Female |
50 |
50 |
16 |
>1:8,000 |
>1:8,000 |
>1:8,000 |
>1:8,000 |
1:1,000 |
1:1,000 |
|
Patient 6 |
Female |
48 |
48 |
16 |
- |
- |
1:8,000 |
1:8,000 |
- |
- |
|
Patient 7 |
Female |
53 |
60 |
18 |
- |
- |
>1:8,000 |
1:8,000 |
- |
- |
|
Patient 8 |
Female |
57 |
60 |
31 |
>1:8,000 |
1:4,000 |
1:4,000 |
1:1,000 |
1:8,000 |
1:8,000 |
|
Patient 9 |
Female |
53 |
62 |
32 |
1:4,000 |
1:500 |
1:16,000 |
1:500 |
- |
- |
|
Patient 10 |
Female |
59 |
60 |
22 |
1:2,000 |
1:2,000 |
1:1000 |
1:1,000 |
- |
- |
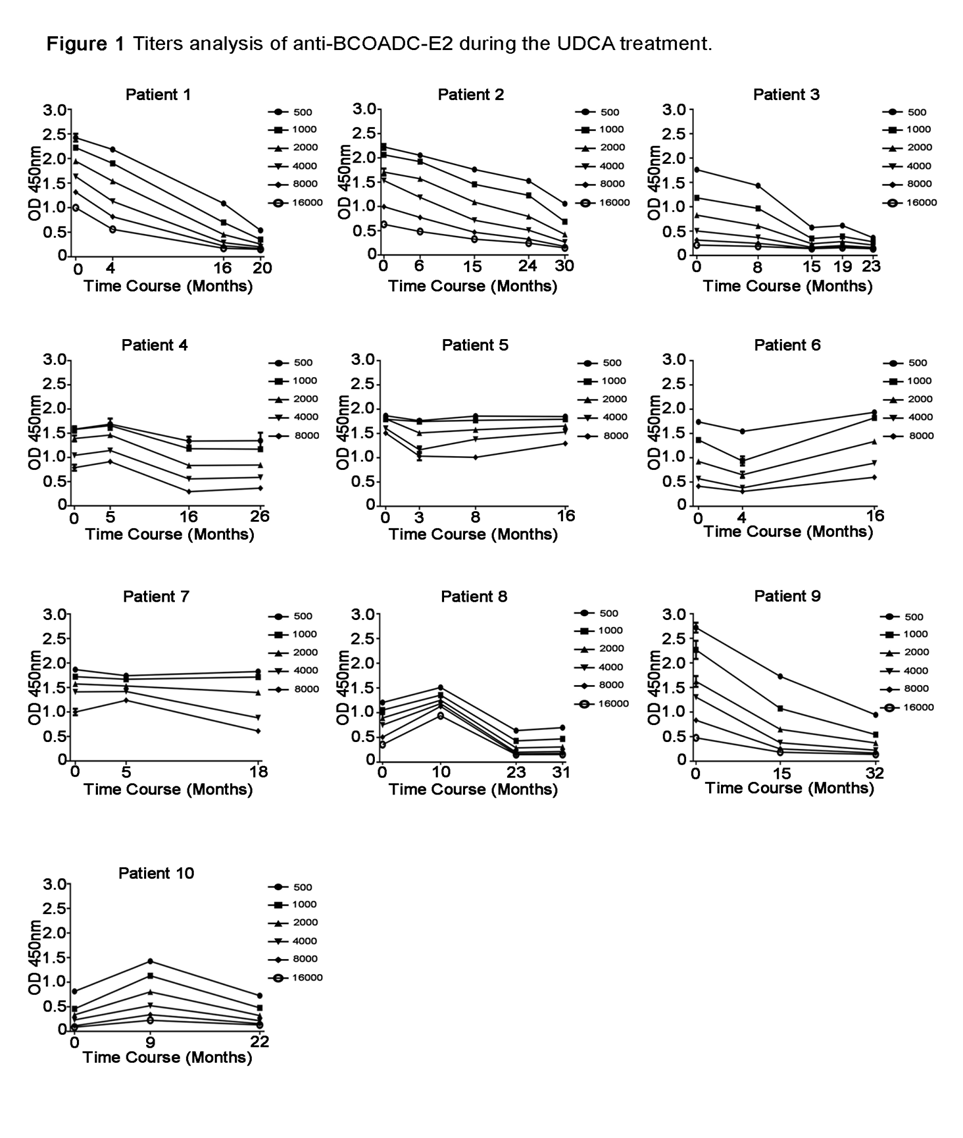
Titers analysis of anti-BCOADC-E2 during the UDCA treatment. Serum samples of the same patients were collected at different time points during UDCA treatment. A series of dilution of each serum sample at different time point were analyzed in triplicates in the same plate per antibody. Optical Density (OD450) value of serum samples of ten PBC patients before (0 month) and after UDCA treatment were plotted against the time after treatment (months). Each sample was analyzed in duplicates or triplicates. Data are represented as mean ± SD.
Titer analysis of AMAs during UDCA treatment
In the course of evaluating our AMA antibody ELISA assay, we unexpectedly found inconsistent anti-BCOADC-E2 results in a patient's blood samples collected at two different times. To assess whether anti-BCOADC-E2 titer can be affected by UDCA treatment, we performed a semi-quantitative analysis of AMA titers in PBC patients during the course of UDCA treatment. In ten initially anti-BCOADC-E2-positive patients studied, seven were positive for anti-PDC-E2 prior to treatment, and four were positive for anti-OGDC-E2 (
Discussion
PDC-E2 was first identified and cloned in 1987 by Gershwin and his colleagues as the main M2 antigenic component of AMA. Later, other members of the 2-OADC complex (BCOADC-E2 and OGDC-E2) were confirmed as constituents of the ‘M2’ family of mitochondrial antigens5, 11. Antibodies to PDC-E2 are the most frequently detected antibodies in Chinese PBC patients, with prevalence similar to that observed in Caucasian PBC patients. The prevalence of anti-BCOADC-E2 is much higher in Chinese PBC patients (84.21%) than in Caucasian PBC patients (64.32%)10. Our results indicate ethnic or geographical variations in the prevalence of AMA antibodies against PDC-E2, BCOADC-E2 and OGDC-E2.
Currently, possible reasons for the higher anti-BCOADC-E2 positivity in Chinese PBC patients may include the high prevalence of hepatitis virus infection in China, limited access to AMA testing in non-metropolitan clinics, and inadequate medical training among clinicians in rural areas, which may lead to delayed or missed diagnoses among many PBC patients12. Due to financial difficulties, many PBC patients cannot afford UDCA treatment and opt for herbal treatments13. These factors may increase the percentage of patients without proper treatment, thus contributing to higher anti-BCOADC-E2 positivity.
In this study, we compared the initial biochemical results of PBC patients with their AMA status. As reported previously by the Gershwin group, anti-PDC-E2 positivity did not correlate with any biochemical index14. However, surprisingly, we found significant association of anti-BCOADC-E2 status with bilirubin concentration. PBC patients positive for anti-BCOADC-E2 antibody showed increased initial total bilirubin and direct bilirubin concentration in the blood, compared to patients negative for anti-BCOADC-E2 antibody. Although our cohort is large and the statistical association is robust, further validation in independent datasets is needed to confirm the generalizability of this finding.
We further performed semi-quantitative analysis of three AMA antibody concentrations during the UDCA treatment. To our surprise, steady decrease in anti-BCOADC-E2 titer was observed in five out of ten patients. After 15–30 months, two patients became negative for anti-BCOADC-E2. A previous study by the Gershwin group also analyzed these three antibodies in 20 patients over 7–28 years. They found no significant changes in antibody titers for PDC-E2, BCOADC-E2 and OGDC-E2 between sera collected at the beginning and end of observation. However, a few significant changes were observed in the study, in which the anti-IgG and anti-IgM antibodies to BCOADC-E2 showed a decrease, although authors did not elaborate in detail14. In their report, the authors did not mention clearly whether the initial patient samples were collected at the beginning of the UDCA treatment. Different from their report, the initial blood samples analyzed in our time course analysis were collected when AMA was first found to be positive in the patient and/or within the first month of initial UDCA treatment. Blood samples in our study were collected in a relatively short period (15–30 months) after disease diagnosis and UDCA treatment. Although our findings show a reduction in anti-BCOADC-E2 titers in some PBC patients during the early stage of UDCA treatment, the mechanisms underlying this immunological change remain unclear. UDCA is known to exert anti-inflammatory and immunomodulatory effects, including improved bile flow and reduced hepatocyte stress, which may indirectly attenuate autoimmune responses. It is possible that these effects contribute to the observed decline in autoantibody titers, though further mechanistic studies are needed.
Although the titer analysis of autoantibodies during UDCA treatment consisted of ten patients, multiple longitudinal samples were collected per individual (3–5 time points each), resulting in a total of approximately 38 samples. This repeated-measures design enabled us to assess within-patient changes over time, providing richer insights into the dynamics of change in titer during UDCA response. Nevertheless, we acknowledge that the limited number of patients restricts generalizability, and the observed trends in antibody changes post-UDCA treatment are preliminary and require confirmation in larger, prospective studies.
The exact role of AMA in the immunopathology and pathogenesis of PBC remains obscure. Some investigators believe that serum AMA is not linked to the progression of PBC, as the AMA titer does not change significantly over the course of the disease15. In our study, we report for the first time that one of the PBC-specific AMA antibodies, BCOADC-E2, showed a significant decrease in antibody titer during the early stage of UDCA treatment. From the limited number of patients studied, we did not find any correlation between the decrease or loss of anti-BCOADC-E2 and changes in specific biochemical indices (data not shown).
Conclusions
In conclusion, PBC-specific AMAs other than anti-PDC-E2 were evaluated for the first time in Chinese PBC cohorts. Higher prevalence of anti-BCOADC-E2 and OGDC-E2 antibodies were observed. A significant correlation between anti-BCOADC-E2 status and initial serum bilirubin concentration of PBC patients was found in Chinese PBC patients. Loss or significant decrease in anti-BCOADC-E2 antibody was found in half of PBC patients after the UDCA treatment. Further studies need to be performed to analyze the clinical significance.
Abbreviations
ALB - albumin, ALP - alkaline phosphatase, ALT - alanine aminotransferase, AMA - anti-mitochondrial antibodies, ANA - anti-nuclear antibodies, AST - aspartate aminotransferase, BA - Barcelona criteria, BCOADC - branched chain 2-oxo-acid dehydrogenase complex, DB - direct bilirubin, E2 - enzyme subunit, GGT - gamma-glutamyl transferase, OGDC - 2-oxo-glutaric acid dehydrogenase complex, PA - Paris I criteria, PBC - primary biliary cholangitis, PDC - pyruvate dehydrogenase complex, SD - standard deviation, TB - total bilirubin, TP - total protein, UDCA - ursodeoxycholic acid
Acknowledgments
We are indebted to the patients with PBC who participated in this study. We thank all participating members of The Jiangsu Provincial PBC Collaboration Group for providing patient samples and clinical information.
Author’s contributions
Jawed R performed experimental work; Jawed R performed statistical analysis; Jawed R prepared and revised the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81270295; No. 81770381, Key R&D Program of Jiangsu Province (SBE2017740378), the Natural Science Foundation of Jiangsu Province (No. SBK2015020697), and the Fundamental Research Funds for the Central Universities.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the ethics committee of southeast university hospital and in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from each patient prior to screening on the approved informed consent form.
Consent for publication
Written informed consent was obtained from the patient’s mother for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

